News
Massive Bio Has Onboarded Over 120,000 Cancer Patients to Find Their Clinical Trial
Discover The Latest Lung Cancer Treatments!
Are you a patient diagnosed with Non-Small Cell Lung Cancer who is seeking innovative treatment options? Massive Bio can help you to access the latest therapies with no-cost clinical research matching.
Start Your JourneyCompliant with

You can receive a guidebook with information about lung cancer by filling out the form.
Non-Small Cell Lung Cancer Treatments!
Massive Bio’s advanced Artificial Intlligence system will match and suggest a range of potential lung cancer clinical trial options for your unique medical situation. Our Patient Relations Coordinator will evaluate your submission and reach out to you to discuss your eligibility for our lung cancer clinical research studies.
Lung cancer is the leading cause of cancer-related deaths worldwide. Non-small cell lung cancer (NSCLC) is the most common type, accounting for 85% of cases. The 5-year survival rate for NSCLC ranges from 16% to 25%. Around 30% of newly diagnosed NSCLC patients have unresectable Stage III disease.


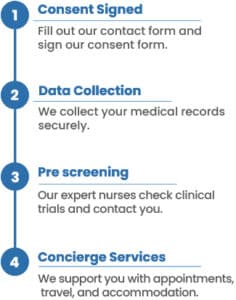
We have an incredible opportunity for you to access a groundbreaking therapy that can revolutionize your journey with stage 3 non-small cell lung cancer. By signing up for our matching program, you’ll be connected to a personalized treatment option specifically tailored to your needs. Here’s how it works:
Diagnosis: If you have been diagnosed with Stage 3, unresectable non-small cell lung cancer (NSCLC), you’re already on the path to potentially innovative treatments. This path may be clearer if you are ALK-positive or ROS1-positive, you’re one step closer to finding a potential breakthrough in your treatment.
Innovative Therapy: Clinical trial therapies include the latest advancements in medical science and technology to target your specific lung cancer. This includes treatments for advanced and inoperable lung cancer, offering solutions where others might not.
Personalized Match: We understand that every patient is unique, and that’s why we focus on matching you with a treatment plan that aligns with your specific requirements and medical history, whether you’re dealing with non-small cell lung cancer or other forms of lung cancer.
Expert Guidance: Throughout your journey, even if you’re dealing with alk positive lung cancer or ros1 positive lung cancer, our experienced team of medical professionals will be by your side, providing guidance, support, and answering any questions you may have.
This study aims to assess the effectiveness and safety of various treatments in patients with locally advanced, unresectable Stage III non-small cell lung cancer (NSCLC). The selection of therapies will be based on the biomarker status determined through tissue-based testing. The main goal is to evaluate the outcomes of different treatments in this specific patient population.
By signing up for our matching program, you’re taking an active role in exploring new possibilities and gaining access to an innovative therapy that can potentially transform your life. Don’t miss out on this opportunity to enhance your lung cancer treatment.
Please note: To qualify for our treatments, it’s essential that you have undergone an NGS test showing an ALK+ or ROS1+ result; if you haven’t, we can arrange a free NGS test for individuals diagnosed with certain conditions, helping pave the way towards potential treatment options.
We combine the power of technology with our dedicated team of medical providers to find you the best treatment options available.
Access the free matching toolA Clinical Trial May Be an Option for You
If you have stage III NSCLC and testing indicates that you are positive for ALK or ROS1, Massive Bio can help you access cutting-edge treatments that are being studied in clinical trials. That includes experimental therapies and existing medicines that are being used in new ways. Patients who enroll in clinical trials receive innovative treatments and high-quality care under the direction of doctors, nurses, and other healthcare professionals who are participating in research to discover new therapies for cancer. Patients can gain access to promising drugs and new treatments long before they’re made available to the public. If you have ALK or ROS1 NSCLC, we’re here to help. If you aren’t sure if you have a gene mutation linked to cancer, that’s okay. Additional testing can help you determine your exact diagnosis.
Talk to us. Our nurses and patient relations coordinators are here and happy to help you. You are not alone in this fight.
Call +1 844 627 7246What is a Non-Small Cell Lung Cancer clinical trial?
Clinical trials test the latest scientific advancements in Non-Small Cell Lung Cancer treatment. Patients who choose to enroll in trials can receive cutting-edge treatment and high-quality care under the direction of scientists, doctors, and researchers. Non-Small Cell Lung Cancer patients might gain access to promising drugs and innovative treatments long before they're made available to the public.

You are not alone
You can have an experienced team with you
What Are Clinical Trials?
Cancer is an unfortunate reality that touches most of us at some point in our lives. If you or a loved one has cancer, you may have heard or read that clinical trials could offer access to innovative new treatments. But what exactly is a clinical trial? In this video, Massive Bio co-founder Arturo Loaiza-Bonilla, MD, explains how clinical trials work, what to expect if you enroll in one, and why a clinical trial can be an important treatment option for many cancer patients.
___
The Other Sources:
-
Why should I trust Massive Bio?
Why should I trust Massive Bio?
Massive Bio has provided health to more than 120,000 cancer patients in 25 countries across three continents. It collaborates with over 80 global partners. In 2022, Massive Bio became part of the Precision Cancer Consortium (PCC), alongside major companies such as AstraZeneca, Bayer, Eli Lilly & Company, GSK, Johnson & Johnson/Janssen, Novartis and Roche.
-
What is the process?
What is the process?
To find the best clinical research studies for you, we need your medical history and consent. You can provide this consent by filling out the form on this page and the following pages. Once you’ve done that, our patient relations coordinator will contact you to discuss the details and provide further information for clinical research matching report.
-
Why do I have to provide my medical records to enroll in a clinical trial?
Why do I have to provide my medical records to enroll in a clinical trial?
To enroll in clinical trial, you must meet highly specific criteria that’s established by the researchers who are conducting the investigation. That includes detailed information about type of cancer, treatment history, response to treatment, and other data that is collected in medical records.
-
What should I do if I don’t have my medical records?
What should I do if I don’t have my medical records?
If you are being treated for cancer or any other disease, your doctor should have a complete record of your medical care, including specific information about what form of the disease you have and what treatments you have received. Your patient relations coordinator will contact you and inform you about the details.
-
What are the costs associated with Massive Bio’s services?
What are the costs associated with Massive Bio’s services?
Massive Bio provides its services to the patients and their doctors at no cost—you won’t have to pay anything to receive a clinical-research matching report. There are no hidden costs involved.
-
How does Massive Bio protect my personal information?
How does Massive Bio protect my personal information?
Massive Bio strictly adheres to all HIPAA guidelines and international regulations focused on maintaining your privacy. We take extra measures to secure your personal information, ensuring it is protected beyond the mandatory requirements.
-
Where can I find clinical research studies in my area?
Where can I find clinical research studies in my area?
Your doctor may know of a clinical research study being conducted in your area that’s recruiting participants and is right for you. However, Massive Bio uses its artificial intelligence-powered platform to match patients to clinical research studies of treatments that give you the best chance of a positive outcome and are being conducted in a geographical location that makes sense for you.
-
Can I continue seeing my doctor or oncologist while also taking services of Massive Bio?
Can I continue seeing my doctor or oncologist while also taking services of Massive Bio?
Yes, Massive Bio keeps your doctor up to date on your status throughout your participation.






