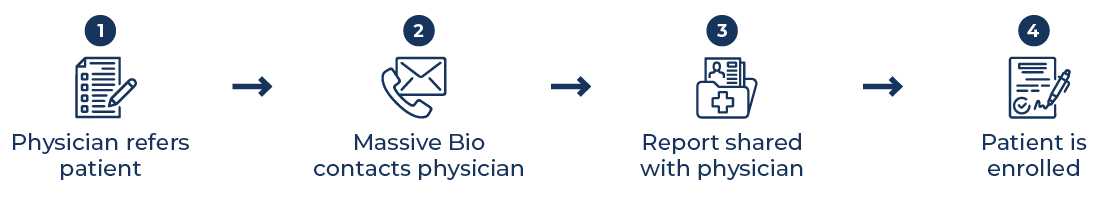
Our Physician Process

AI Driven Platform
Oncology Trial Matching with Massive Bio
Massive Bio offers free trial services, granting access to potential life-saving treatments. Join us to explore diverse treatment options and improve your prognosis and quality
Get StartedAllow artificial intelligence to streamline your process, enabling your patients to reach cutting-edge trials, while enhancing your trial oversight and clinical results.
Refer Your Patient
Massive Bio has provided health to more than 120,000 cancer patients in 25 countries across three continents. It collaborates with over 80 global partners. In 2022, Massive Bio became part of the Precision Cancer Consortium (PCC), alongside major companies such as AstraZeneca, Bayer, Eli Lilly & Company, GSK, Johnson & Johnson/Janssen, Novartis and Roche.
Many clinical patients received
successful treatments
successful treatments










Many clinical patients received
successful treatments
successful treatments
There were times when I felt Massive Bio was the only champion I had working toward my acceptance into the clinical trial!
Cancer is a word that everyone cringes at. You need services like Massive Bio to keep hope.
The initial report and clinical trial recommendations was excellent.
I have the confidence of knowing that my plan is based on the most current science as it applies to my cancer type.
Massive Bio was quick and insightful in finding me the best clinical trial option.

John D.
Patient - United States of America

Cindy O.
Patient – United States of America

Michael L.
Patient – United States of America

Steve W.
Patient – United States of America

Kelly R.
Patient - United States of America