News
Massive Bio Has Onboarded Over 120,000 Cancer Patients to Find Their Clinical Trial
Exploring Clinical Trial Opportunities for Solid Tumor Cancers
The treatment of cancerous solid tumors often leads to many questions, but there's a rising sense of optimism. Our service bridges the gap to the most current clinical trials, focusing on advanced solid tumors, including Stage 3 and metastatic Stage 4 cancers. We streamline the pathway for patients to locate and enroll in these innovative trials, granting them opportunities to explore the forefront of therapeutic advancements.
Compliant with

You can receive a guidebook with information about genomic testing by filling out the form.
The Most Recent Advances in Cancer Treatment Options
Our matching system links you to the latest clinical trials, offering new opportunities for those fighting solid tumors. You’ll find detailed information on clinical trials for all stages of these tumors, with particular attention to advanced stages like Stage III and Stage IV. These trials feature innovative treatment approaches that are not widely available, concentrating on specific biomarkers associated with your diagnosis. This is your opportunity to take an active role in managing your condition with our expert guidance and support.
How our system works:
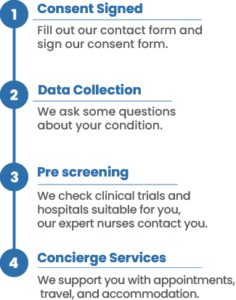
After reviewing your medical records and running the information through our AI powered system, we identify clinical trials you may be eligible for. Not only that, we also provide a concierge service that will allow for a smooth transition into the clinical trial.


Our team of specialized nurses and physicians will make sure that every detail of your participation in the trial is taken care of before, during and after your enrollment.
All the services provided are completely free for you and your treating oncologist.
The Role of Clinical Trials in
Solid Tumors
Clinical trials play a crucial role in the treatment of stage 3 and stage 4 solid tumor cancer for several reasons:
1. Access to New Treatments: Clinical trials offer patients a chance to try new treatments that could work better, especially for serious illnesses like advanced cancer, where usual treatments might not work well.
2. Improving Outcomes: By participating in clinical trials, patients contribute to knowledge that could improve survival rates and quality of life for future cancer patients.
3. Comprehensive Care: Patients in clinical trials often receive a high level of care and are closely monitored by a dedicated team of healthcare professionals and researchers.
4. Hope for the Future: For many patients with advanced cancer, participating in a clinical trial provides hope—both for themselves and the possibility that their participation will help others in the future.
5. Advancing Research: These trials are essential for research, helping scientists understand how new treatments work and for whom they work best. This can lead to the development of more effective treatment strategies and personalized medicine.
Understanding the disease
What are solid tumors?
Solid tumor cancers refer to a group of cancers that form a mass or lump of abnormal cells, unlike liquid cancers such as leukemia which affect the blood cells and circulate through the body fluids. These solid tumors can arise in any organ or tissue in the body and are categorized based on the type of cells that form them. The main types of solid tumors are:
1. Carcinomas: The most common type, originating in the skin or tissues that line or cover internal organs. Examples include breast, lung, prostate, and colorectal cancers.
2. Sarcomas: Arising from connective or supportive tissues such as bone, cartilage, fat, muscle, or blood vessels.
3. Gliomas: Developing in the brain and spinal cord, originating from glial cells.
4. Lymphomas: Though typically considered a blood cancer, some lymphomas can form solid tumor masses, particularly in the lymph nodes.
Each type of solid tumor is unique in its growth behavior, how it spreads (metastasizes), and how it responds to treatment. Treatment can include surgery, radiation therapy, chemotherapy, targeted therapy, immunotherapy, or a combination of these, depending on the type, stage, and location of the tumor, as well as the patient’s overall health.
NGS testing and clinical trials
Next-Generation Sequencing (NGS) is vital in linking patients with specific genetic mutations in their cancers to clinical trials for targeted treatments. This approach, part of personalized medicine, increases the effectiveness and success rates of these trials by ensuring patients receive therapies most likely to work for their cancer type.
In solid tumors, certain genetic alterations are commonly observed, which contribute to the development and progression of the disease. Some of the key genes frequently altered in solid tumors include:
1. ROS 1: a genetic alteration that serves as a critical factor in the development of certain cancers. It is a receptor tyrosine kinase, a protein crucial for cell growth and division. When the ROS1 gene is altered or rearranged, it can lead to uncontrollable cell growth, resulting in cancer.
The primary cancers associated with ROS1 gene alterations include:
• Non-Small Cell Lung Cancer (NSCLC): The most common type associated with ROS1 alterations. These genetic changes are particularly significant in NSCLC, especially among specific groups like never-smokers and younger patients.
• Additionally, ROS1 alterations have been observed in other cancers, albeit less frequently:
1. Glioblastomas (Brain Cancers)
2. Ovarian Cancer
3. Gastric Cancer
4. Colorectal Cancer
The presence of ROS1 alterations in these cancers is important for treatment, as targeted therapies known as ROS1 inhibitors can be highly effective. These drugs specifically inhibit the ROS1 protein’s activity, helping to control the growth of cancer cells. This targeted approach has significantly improved the treatment outcomes for patients with ROS1-positive cancers, particularly in non-small cell lung cancer.
2. NTRK 1/2/3: This refers to genes that can be involved in the development of various cancers through genetic alterations. These genes encode for the TRK (tropomyosin receptor kinase) proteins: TRKA, TRKB, and TRKC, respectively. Alterations in these genes, such as fusions, can lead to the continuous activation of these kinases, promoting uncontrolled cell growth and cancer.
Cancers associated with NTRK gene fusions include:
• Salivary Gland Tumors: These are among the most common cancers associated with NTRK gene fusions.
• Infantile Fibrosarcoma: A rare type of soft tissue cancer in children, often linked to NTRK fusions.
• Thyroid Cancer: Certain types of thyroid cancer, like papillary thyroid carcinoma, can harbor NTRK gene fusions.
• Lung Cancer: NTRK gene fusions are found in a small subset of non-small cell lung cancers.
• Colorectal Cancer: A fraction of colorectal cancers may have NTRK gene fusions.
• Other Cancer Types: NTRK gene fusions have been observed in various other cancers, including some breast cancers, certain brain tumors (gliomas), melanomas, and gastrointestinal stromal tumors (GISTs), although these are less common.
The discovery of NTRK gene fusions in a tumor is significant because it opens the door for targeted therapy using TRK inhibitors. These drugs specifically target the abnormal TRK proteins produced by these gene fusions, offering a more personalized and often more effective treatment approach. The development of TRK inhibitors has been a significant advancement, especially for cancers where NTRK gene fusions are the primary driver of the disease.
3. ALK Fusion: refers to a genetic alteration involving the ALK (anaplastic lymphoma kinase) gene. This alteration typically occurs when the ALK gene fuses with another gene, leading to the production of an abnormal ALK protein that can promote the growth of cancer cells. ALK fusions are significant because they can be targeted by specific drugs known as ALK inhibitors.
Cancers associated with ALK fusions include:
• Non-Small Cell Lung Cancer (NSCLC): ALK fusions are most commonly identified in NSCLC, particularly in a subset of patients who are often younger, non-smokers, or light smokers.
• Anaplastic Large Cell Lymphoma (ALCL): ALK positive ALCL is a type of non-Hodgkin lymphoma where ALK fusions play a crucial role.
• Inflammatory Myofibroblastic Tumor: This is a rare tumor where ALK gene rearrangements are frequently observed.
• Other Cancers: While less common, ALK fusions have also been identified in some cases of colorectal cancer, breast cancer, neuroblastoma (a pediatric cancer), and other rare cancers.
The identification of an ALK fusion in a cancer patient is highly important for treatment planning. ALK inhibitors, which target the abnormal ALK protein, have shown remarkable effectiveness, particularly in lung cancer, leading to significant improvements in treatment outcomes. These targeted therapies provide a more personalized treatment approach, offering benefits like lower toxicity and better response rates compared to conventional chemotherapy.
4. TMB: “Tumor Mutational Burden” (TMB) is not a specific type of genetic alteration like ALK fusions or NTRK gene fusions, but rather a measure used in the field of oncology. TMB quantifies the number of mutations within a tumor’s DNA. A high TMB suggests that the cancer cells have many mutations, which can influence the behavior of the cancer and its response to certain treatments, particularly immunotherapy.
Cancers where TMB is particularly relevant include:
• Melanoma: Known for having high TMB, partly due to UV radiation causing DNA damage.
• Lung Cancer: Both small cell and non-small cell lung cancers often have high TMB, especially in smokers due to exposure to carcinogens in tobacco smoke.
• Bladder Cancer: This cancer type frequently exhibits a high TMB.
• Colorectal Cancer: Especially those that are microsatellite instability-high (MSI-H) or have mismatch repair deficiency (dMMR), which are conditions that lead to a high TMB.
• Head and Neck Cancers: These cancers, particularly those associated with human papillomavirus (HPV), can show a high TMB.
• Other Cancer Types: A high TMB can be found in various other cancers, though less commonly.
High TMB can be an important biomarker for the effectiveness of immunotherapies like checkpoint inhibitors, as tumors with more mutations may present more targets (neoantigens) for the immune system to recognize and attack. This has led to the use of TMB as a biomarker to predict which patients might benefit most from immunotherapies. However, the relevance of TMB can vary widely among different cancer types and individual patients.
5. AKT 1/2/3: are genes that encode for the AKT proteins, which are part of a cell signaling pathway important for regulating cell growth, survival, and metabolism. Alterations or mutations in these genes can contribute to the development and progression of various cancers by promoting cell proliferation and inhibiting programmed cell death (apoptosis).
Cancers associated with alterations in AKT1, AKT2, and AKT3 include:
• Breast Cancer: AKT1 mutations are particularly noted in certain subtypes of breast cancer.
• Ovarian Cancer: Alterations in AKT genes have been observed in ovarian cancer, contributing to tumor development and resistance to treatment.
• Lung Cancer: Mutations in AKT genes can be involved in non-small cell lung cancer (NSCLC).
• Colorectal Cancer: AKT mutations or overactivation can play a role in colorectal cancer progression.
• Prostate Cancer: AKT signaling is often upregulated in prostate cancer and is associated with disease progression and poor prognosis.
• Endometrial Cancer: AKT1 mutations are known to occur in a subset of endometrial cancers.
• Glioblastoma: This aggressive brain tumor can exhibit alterations in AKT signaling.
• Other Cancers: Alterations in the AKT pathway can also be found in various other cancer types, although less frequently.
It’s important to note that while AKT mutations and the resultant pathway alterations are implicated in cancer development, they are just one part of a complex network of molecular changes driving oncogenesis. The presence of AKT mutations can influence the choice of targeted therapies in cancer treatment, as drugs that inhibit AKT signaling might be effective in tumors with these specific alterations. Research into AKT inhibitors and their role in cancer therapy is ongoing.
6. HER 2: (Human Epidermal Growth Factor Receptor 2) is a gene that plays a key role in the development of certain types of cancer. The HER2 gene produces a protein receptor on the surface of cells that can control growth and division. When the HER2 gene is amplified or overexpressed, it leads to the overproduction of this receptor, causing cells to divide and grow uncontrollably, which can lead to cancer.
Cancers associated with HER2 amplification or overexpression include:
• Breast Cancer: HER2-positive breast cancer is a subtype characterized by the overexpression of the HER2 protein. This type of breast cancer tends to be more aggressive than other types.
• Gastric Cancer (including Gastroesophageal Junction Cancer): HER2 overexpression is also found in some gastric cancers, influencing the growth of these cancer cells.
• Ovarian Cancer: A proportion of ovarian cancers show HER2 amplification.
• Bladder Cancer: HER2 can be overexpressed in a subset of bladder cancer cases.
• Esophageal Cancer: HER2 overexpression is observed in some cases of esophageal cancer.
• Other Cancers: Although less common, HER2 amplification or overexpression has been identified in other types of cancers, including colorectal, lung, endometrial, and salivary gland cancers.
The identification of HER2 status in cancers, especially breast and gastric cancers, is crucial because it guides treatment strategies. HER2-positive cancers often respond to targeted therapies that specifically inhibit the HER2 receptor, such as trastuzumab (Herceptin) and other HER2-targeted agents. These treatments have significantly improved outcomes for patients with HER2-positive cancers. The presence of HER2 overexpression or amplification is therefore a key factor in the diagnosis and treatment planning for patients with these cancer types.
7. PIK3CA: is a gene that encodes for a subunit of the enzyme PI3K (phosphoinositide 3-kinase), which is involved in cell growth, proliferation, differentiation, and survival. Mutations in the PIK3CA gene can lead to the overactivation of the PI3K pathway, contributing to the development and progression of various cancers by promoting uncontrolled cell growth and resistance to cell death.
Cancers associated with PIK3CA mutations include:
• Breast Cancer: PIK3CA mutations are common in breast cancer, particularly in hormone receptor-positive and HER2-positive subtypes.
• Endometrial Cancer: These mutations are frequently observed in endometrial cancer and are associated with certain subtypes of the disease.
• Colorectal Cancer: PIK3CA mutations can be found in a significant proportion of colorectal cancer cases, often associated with other genetic alterations.
• Ovarian Cancer: Mutations in PIK3CA are present in some ovarian cancer cases, contributing to tumor growth.
• Gastric Cancer: PIK3CA mutations are also found in gastric cancer, affecting the aggressiveness and treatment response of the tumors.
• Head and Neck Cancers: Particularly in squamous cell carcinomas of the head and neck, PIK3CA mutations play a role in cancer development.
• Other Cancers: PIK3CA mutations can occur in various other cancer types, though less frequently, including glioblastomas, lung cancer, and others.
The presence of PIK3CA mutations in these cancers is significant for treatment considerations. Targeted therapies that specifically inhibit the PI3K pathway, such as PI3K inhibitors, are being developed and used in the treatment of cancers with PIK3CA mutations. These targeted treatments can offer a more personalized approach to cancer therapy, with the potential for improved efficacy and reduced toxicity compared to traditional chemotherapies. The identification of PIK3CA mutations helps in tailoring treatment plans to better suit the individual genetic profile of a patient’s cancer.
8. BRAF: is a gene that encodes for a protein kinase involved in sending signals inside cells, which are crucial for cell growth and division. Mutations in the BRAF gene, most notably the V600E mutation, lead to the activation of the MAPK/ERK signaling pathway, promoting uncontrolled cell growth and the development of tumors.
Cancers associated with BRAF mutations include:
• Melanoma: BRAF mutations, especially the V600E mutation, are highly prevalent in melanoma. This mutation is found in about 40-60% of melanoma cases, making it a significant target for treatment.
• Colorectal Cancer: A significant proportion of colorectal cancers have BRAF mutations, which are associated with a more aggressive disease and poorer prognosis.
• Thyroid Cancer: BRAF mutations are common in certain types of thyroid cancer, particularly papillary thyroid carcinoma.
• Non-Small Cell Lung Cancer (NSCLC): BRAF mutations are present in a subset of NSCLC patients.
• Ovarian Cancer: While less common, BRAF mutations can be found in some cases of ovarian cancer.
• Other Cancers: BRAF mutations also occur in other cancer types, though less frequently, such as hairy cell leukemia, gliomas, and certain types of sarcomas.
The identification of a BRAF mutation in a cancer patient is crucial as it influences treatment strategies. Targeted therapies, specifically BRAF inhibitors (like vemurafenib and dabrafenib), have been developed for cancers harboring these mutations. These drugs specifically inhibit the mutated BRAF protein, effectively blocking the aberrant signaling pathway that promotes tumor growth. For certain cancers like metastatic melanoma, the combination of BRAF and MEK inhibitors has significantly improved treatment outcomes. The presence of BRAF mutations, therefore, plays a vital role in the diagnosis and targeted treatment of these cancers.
9. RET: Alterations in the RET gene, which encodes for a receptor tyrosine kinase involved in cell growth, maturation, and differentiation. Mutations and rearrangements in the RET gene can lead to the development of various cancers by causing abnormal cell growth and proliferation.
Cancers associated with RET mutations and rearrangements include:
• Thyroid Cancer: RET mutations are particularly significant in thyroid cancer. They are commonly seen in medullary thyroid carcinoma (MTC), where they can be either inherited or sporadic. RET rearrangements (known as RET fusions) are also found in papillary thyroid carcinoma.
• Lung Cancer: RET fusions are identified in a small percentage of non-small cell lung cancer (NSCLC) cases, particularly in adenocarcinomas.
• Other Cancer Types: While less common, RET alterations can occur in other cancers such as colorectal cancer, breast cancer, and pancreatic cancer.
The presence of RET mutations or fusions in these cancers is important for treatment decisions. The development of RET inhibitors, targeted therapies specifically designed to block the activity of the mutated RET protein, has been a significant advancement in the treatment of cancers with these genetic alterations. These inhibitors have shown effectiveness in treating cancers, particularly thyroid and lung cancers, with RET mutations or fusions, offering a more personalized and often more effective treatment option compared to traditional chemotherapy. The identification of RET alterations, therefore, is crucial in the diagnosis and targeted treatment approach for patients with these specific cancer types.
10. KRAS: gene that plays a critical role in cell signaling pathways related to cell growth, differentiation, and survival. Mutations in the KRAS gene can lead to the production of a continuously active KRAS protein, which can cause uncontrolled cell growth and contribute to the development of cancer.
Cancers associated with KRAS mutations include:
• Colorectal Cancer: KRAS mutations are common in colorectal cancer, particularly in metastatic cases, and they are known to affect treatment response and prognosis.
• Lung Cancer: Specifically, in non-small cell lung cancer (NSCLC), KRAS mutations are a significant genetic alteration, especially in adenocarcinomas.
• Pancreatic Cancer: KRAS mutations are highly prevalent in pancreatic cancer, being one of the most commonly mutated genes in this cancer type.
• Ovarian Cancer: Some types of ovarian cancer, particularly mucinous adenocarcinomas, can harbor KRAS mutations.
• Other Cancers: KRAS mutations also occur, though less frequently, in other cancer types such as endometrial cancer, gastric cancer, and bile duct cancer (cholangiocarcinoma).
The presence of KRAS mutations in a tumor is important because it influences treatment decisions and can affect the prognosis of the patient. Traditionally, cancers with KRAS mutations were considered resistant to certain types of therapy, particularly EGFR inhibitors in colorectal and lung cancers. However, recent developments in targeted therapies have led to the approval of specific inhibitors that can target KRAS G12C mutations, a specific subset of KRAS mutations, offering new treatment options for patients with these mutations. The identification of KRAS mutations is thus crucial in the diagnosis, prognosis, and treatment planning for patients with these cancer types.
11. ATM: gene that plays a crucial role in the DNA damage response, particularly in the repair of double-strand breaks. Mutations in the ATM gene can lead to impaired DNA repair mechanisms, contributing to genomic instability and the development of various cancers.
Cancers associated with ATM mutations include:
• Breast Cancer: ATM mutations have been linked to an increased risk of developing breast cancer, particularly in individuals who are carriers of one mutated copy of the gene.
• Pancreatic Cancer: Studies have shown a higher prevalence of ATM mutations in pancreatic cancer patients, impacting the progression and treatment response of the disease.
• Prostate Cancer: Mutations in the ATM gene are associated with an increased risk and aggressive forms of prostate cancer.
• Ovarian Cancer: ATM mutations can also be found in ovarian cancer, contributing to tumor development and progression.
• Leukemia: Specifically, chronic lymphocytic leukemia (CLL) has been associated with ATM mutations.
• Other Cancer Types: While less common, mutations in the ATM gene can also occur in other types of cancers, such as stomach (gastric) cancer, and certain types of lung cancer.
The significance of ATM mutations in cancer is linked to the role of ATM in DNA repair. Loss of proper ATM function can make cells more susceptible to DNA damage, leading to cancer development. In terms of treatment, cancers with ATM mutations may respond differently to certain therapies, especially those that cause DNA damage, such as radiation and some types of chemotherapy. Moreover, the presence of ATM mutations has been explored as a potential biomarker for the effectiveness of PARP inhibitors, a class of drugs used in cancer treatment, particularly in breast and ovarian cancers. The understanding of ATM’s role in cancer continues to evolve, with ongoing research into targeted therapies for cancers harboring ATM mutations.
12. SETD2: refers to the SET Domain Containing 2 gene, which is known for its role in chromatin remodeling and gene regulation. SETD2 is a histone methyltransferase, an enzyme that modifies histones (proteins around which DNA is wrapped). Specifically, SETD2 trimethylates the 36th lysine residue of histone H3 (H3K36me3), a modification crucial for the correct packaging and regulation of DNA in cells.
Mutations or alterations in the SETD2 gene are implicated in various types of cancer and other diseases, as they can lead to disruptions in chromatin structure and gene expression. These include:
• Renal Cell Carcinoma (Kidney Cancer): Loss of function mutations in SETD2 are frequently observed in clear cell renal cell carcinoma.
• Leukemia: Some forms of leukemia, such as acute lymphoblastic leukemia (ALL), may involve alterations in the SETD2 gene.
• Other Cancers: While less common, mutations in SETD2 have been identified in other types of cancers, including certain brain cancers and breast cancers.
The function of SETD2 in normal cell biology and its implications in cancer and other diseases are areas of active research. Understanding how SETD2 mutations affect cancer development and progression can potentially lead to the development of new therapeutic strategies targeting these pathways.
We combine the power of technology with our dedicated team of medical providers to find you the best treatment options available.
Access the free matching toolHow Massive Bio Helps Patients With Biomarker-Based Cancers
Massive Bio offers a quick, easy, and FREE way to find clinical trials for patients like you. With our unique clinical trial matching system and compassionate team, Massive Bio can rapidly match you to clinical trials of new therapies. If you aren’t sure if you have a biomarker-based cancer, that’s okay. Additional testing can help determine your exact diagnosis.
Talk to us. Our nurses and patient relations coordinators are here and happy to help you. You are not alone in this fight.
Call +1 844 627 7246What is a clinical trial?
Clinical trials test the latest scientific advancements in cancer treatment. Patients who choose to enroll in trials can receive cutting-edge treatment and high-quality care under the direction of scientists, doctors, and researchers. Cancer patients can gain access to promising drugs and innovative treatments long before they're made available to the public.
What Are Clinical Trials?
Cancer is an unfortunate reality that touches most of us at some point in our lives. If you or a loved one has cancer, you may have heard or read that clinical trials could offer access to innovative new treatments. But what exactly is a clinical trial? In this video, Massive Bio co-founder Arturo Loaiza-Bonilla, MD, explains how clinical trials work, what to expect if you enroll in one, and why a clinical trial can be an important treatment option for many cancer patients.
We dream of the day when cancer disappears from our lives. Massive Bio is working tirelessly on achieving that goal.
-
Why should I trust Massive Bio?
Why should I trust Massive Bio?
Massive Bio has provided health to more than 120,000 cancer patients in 25 countries across three continents. It collaborates with over 80 global partners. In 2022, Massive Bio became part of the Precision Cancer Consortium (PCC), alongside major companies such as AstraZeneca, Bayer, Eli Lilly & Company, GSK, Johnson & Johnson/Janssen, Novartis and Roche.
-
What is the process?
What is the process?
To find the best clinical research studies for you, we need your medical history and consent. You can provide this consent by filling out the form on this page and the following pages. Once you’ve done that, our patient relations coordinator will contact you to discuss the details and provide further information for clinical research matching report.
-
Why do I have to provide my medical records to enroll in a clinical trial?
Why do I have to provide my medical records to enroll in a clinical trial?
To enroll in clinical trial, you must meet highly specific criteria that’s established by the researchers who are conducting the investigation. That includes detailed information about type of cancer, treatment history, response to treatment, and other data that is collected in medical records.
-
What should I do if I don’t have my medical records?
What should I do if I don’t have my medical records?
If you are being treated for cancer or any other disease, your doctor should have a complete record of your medical care, including specific information about what form of the disease you have and what treatments you have received. Your patient relations coordinator will contact you and inform you about the details.
-
What are the costs associated with Massive Bio’s services?
What are the costs associated with Massive Bio’s services?
Massive Bio provides its services to the patients and their doctors at no cost—you won’t have to pay anything to receive a clinical-research matching report. There are no hidden costs involved.
-
How does Massive Bio protect my personal information?
How does Massive Bio protect my personal information?
Massive Bio strictly adheres to all HIPAA guidelines and international regulations focused on maintaining your privacy. We take extra measures to secure your personal information, ensuring it is protected beyond the mandatory requirements.
-
Where can I find clinical research studies in my area?
Where can I find clinical research studies in my area?
Your doctor may know of a clinical research study being conducted in your area that’s recruiting participants and is right for you. However, Massive Bio uses its artificial intelligence-powered platform to match patients to clinical research studies of treatments that give you the best chance of a positive outcome and are being conducted in a geographical location that makes sense for you.
-
Can I continue seeing my doctor or oncologist while also taking services of Massive Bio?
Can I continue seeing my doctor or oncologist while also taking services of Massive Bio?
Yes, Massive Bio keeps your doctor up to date on your status throughout your participation.






