News
Massive Bio Has Onboarded Over 120,000 Cancer Patients to Find Their Clinical Trial
Tomorrow's Non-Small Cell Lung Cancer Treatment Today
Get ahead of standard treatments for non-small cell lung cancer. Participating in a non-small cell lung cancer clinical trial gives you access to the latest therapies.
Compliant with

You can receive a guidebook with information about lung cancer by filling out the form.
Understanding Your Options for Treating Non-Small Cell Lung Cancer
Finding out that your non-small cell lung cancer has advanced can be upsetting and frightening, but getting your treatment plan in place can help restore a sense of confidence and calm. Your doctor will discuss your treatment options, and the right one for you will depend on a variety of factors, including your age, overall health, and the results of certain tests. What’s more, your doctor will stage your cancer, which is a system for determining the extent of a malignancy. The stage of your malignancy will have an important influence on the choice of non-small cell lung cancer treatment.


The stages of lung cancer run from one 1 to 4. If you have stage 4 lung cancer, that means the malignancy has spread beyond the lungs to other parts of the body. While chemotherapy was once the main option for treating stage 4 lung cancer, newer medicines are now available. For example, targeted therapies are drugs that block or disable specific proteins that cancer cells need to survive. Another new class of drugs, called immunotherapies, work by boosting the body’s immune system, helping it to kill cancer cells. Other new drugs for cervical cancer are being developed in lung cancer clinical trials.
What Are Clinical Trials?
A clinical trial is a research study in which scientists evaluate the effectiveness and safety of new medical treatments. That includes new drugs or novel combinations of drugs for treating cancer and other diseases. Every drug approved by the U.S. Food and Drug Administration had to be tested first in clinical trials.
We combine the power of technology with our dedicated team of medical providers to find you the best treatment options available.
Access the free matching toolHow Massive Bio Helps Patients With Non-Small Cell Lung Cancer
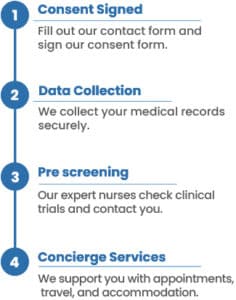
Massive Bio offers a quick, easy, and FREE way to find a non-small cell lung cancer clinical trial. With our unique clinical trial matching system and compassionate team, Massive Bio can rapidly match you to lung cancer clinical trials of new therapies. If you aren’t sure what form of lung cancer you have, that’s okay. Additional testing can help determine your exact diagnosis.
Talk to us. Our nurses and patient relations coordinators are here and happy to help you. You are not alone in this fight.
Call +1 844 627 7246What is a Non-Small Cell Lung Cancer clinical trial?
Clinical trials test the latest scientific advancements in Non-Small Cell Lung Cancer treatment. Patients who choose to enroll in trials can receive cutting-edge treatment and high-quality care under the direction of scientists, doctors, and researchers. Non-Small Cell Lung Cancer patients might gain access to promising drugs and innovative treatments long before they're made available to the public.

You are not alone
You can have an experienced team with you
At Massive Bio, our goal is to ensure that all cancer patients have access to clinical trials of innovative new treatments that offer hope for improved outcomes. Our unique SYNERGY-AI technology rapidly generates a personalized clinical trial matching report, which is FREE to patients and doctors.
ROS1 Positive Lung Cancer
ROS1 positive lung cancer refers to cases of lung cancer in which the patient is positive for a mutation of the ROS1 gene. The ROS1 gene is responsible for making the protein ROS1, which signals cells to grow and reproduce. If a lung cancer patient is ROS1 positive, the cancer is usually more aggressive due to the ROS1 protein causing more abnormal cells to drive the growth of the tumor. However, ROS1 positive patients may be eligible for checkpoint inhibitors for treatment, which block the mutation from causing the cancerous cells to rapidly grow and divide. Although rare, the identification of the ROS1 alteration can play a major role in the patient’s treatment plan. Other than lung cancer, the ROS1 mutation has been identified in the following cancer types:
-Glioblastoma multiforme
-Bile duct cancer
-Ovarian cancer
-Colorectal cancer
Genetic Testing for ROS1
ROS1-positive lung cancer has only been identified in non-small cell lung cancer (NSCLC), and not in small cell lung cancer. Of NSCLC cases, about 2% of them are ROS1 positive. This has led to an increase in testing for NSCLC patients.
In order to be eligible for targeted therapies, cancer patients need a positive test for the ROS1 mutation. Typically, a tissue sample of the tumor is collected by the doctor and it is sent to a laboratory for results. The tests used for the ROS1 mutation include:
-Fluorescence in situ hybridization (FISH): The most common testing method used, locates specific DNA sequences by exposing chromosomes to a probe with a fluorescent molecule attached. A researcher will be able to identify if the ROS1 gene if the fluorescent probe has been bound to it.
-Multiplex real-time PCR assays: Test that covers a range of fusion genes. Inexpensive and quick results compared to other tests. This test uses multiple probes that can be distinguished from the rest of the DNA sequences.
-Immunohistochemistry: Uses antibodies against the ROS1 protein to detect any abnormalities. This test is for screening to diagnose cancers, so it can be used initially to avoid unnecessary FISH tests.
-Next Generation Sequencing (NGS): Looks at the entire human genome in one test to detect any mutation. NGS is the most comprehensive test available, but may be expensive and time consuming compared to other testing methods.
ROS1 Positive Lung Cancer Clinical Trials
The only FDA approved treatments for ROS1 positive lung cancer are Crizotinib (Xalkori) and entrectinib (Rozlytrek). These targeted therapies can locate the abnormalities within cells (mutations) to know which cells to attack. This helps treatment avoid damaging healthy cells to minimize side effects compared to standard treatments.
Common side effects of ROS1 inhibitors include:
-Dizziness
-Diarrhea
-Constipation
-Fatigue
-Changes in vision
There are also ongoing clinical trials evaluating new therapies for ROS1 cancers. There are both clinical trials specifically for the ROS1 mutation, and many others available to treat lung cancer. Talk with your doctor to see if clinical trials could benefit you. Other drugs available for ROS1 lung cancer cases include Lorlatinib and Ceritinib. These therapies are often taken by themselves or in combination with chemotherapy and other treatments.
ALK Fusion Clinical Trials
Anaplastic lymphoma kinase (ALK) fusion is a genetic mutation in the ALK gene that is responsible for making a protein called ALK receptor tyrosine kinase. An ALK fusion is when the rearrangement of the gene EML4 and ALK fuse together, creating an oncogene EML4-ALK. ALK fusions can be a predictive biomarker for the effective use of certain targeted drug therapies, so it is important for patients to know if they have an ALK fusion. Patients without an ALK fusion will likely not respond to targeted ALK inhibitor drug therapies. ALK fusion clinical trials study the ALK inhibiting drugs that are available to treat patients who have this gene alteration.
ALK fusion clinical trials commonly target to treat malignant solid tumors, which are cancerous tumors that do not contain cysts or liquid area. Examples of solid tumors include:
-Sarcomas
-Carcinomas
-Lymphomas
ALK fusion clinical trials are currently studying ALK inhibitor drug therapies. An ALK inhibiting drug inhibits proteins involved in the abnormal growth of tumor cells, which is why these drugs are more effective for patients with this gene alteration. Drug therapies being studied in clinical trials include:
-Alectinib
-Brigatinib
-Crizotinib
-Ceritinib
-Lorlatinib
-Cyclophosphamide
-Ensartinib
-Rituximab
-Doxorubicin
-Nivolumab
Testing for ALK Fusion
Cancer patients will be tested for ALK fusions if their doctor performs Next Generation Sequencing (NGS) to see if they would respond successfully to an ALK inhibitor drug therapy. Genetic testing for cancer patients can be beneficial, as there are many targeted drug therapies for biomarkers other than ALK fusions. Testing for ALK fusions usually coincides with testing for other gene mutations, such as ROS1 and EFGR mutations in non-small cell lung cancer. There are a few methods that are used to test for ALK mutations:
-Next generation sequencing (NGS): detects ALK fusions and the fusion partner gene
-Fluorescent in situ hybridization (FISH): looks for gene rearrangement, and is a common test used to look for ALK fusion
-Immunohistochemistry (IHC): detects the altered ALK protein
-Polymerase chain reaction (PCR): detects known ALK fusions, but not new fusions
Non-Small Cell Lung Cancer Genomic Testing
Non-Small Cell Lung Cancer Genomic Testing helps cancer patients determine which mutations they have inherited that will affect their prognosis.
Non-Small Cell Lung Cancer Genomic Testing expresses the genes displayed by a cancer tumor. Any of these genes may have been mutated. Mutated gene structures help doctors gain insight into tumor behavior and propensity to spread.
What is Non-Small Cell Lung Cancer Genomic Test?
Genomic testing is used to detect changes in the DNA of cancer patients and tumors. Better targeted cancer treatment can be planned, including personalized treatments with the help of genomic tests.
Genetic testing is now an important part of diagnosing and staging patients with non-small cell lung cancer. Some chemotherapy drugs may be more or less effective than others against tumors with certain mutations. Molecular analysis of the tumor helps determine which treatments will benefit the patient the most.
Almost all of the genetic changes in cells are found only in cancer cells, not normal cells. This indicates that it is not possible to pass it on to your children.
Non-Small Cell Lung Cancer Genetic Markers
Currently, genomic abnormalities that are clinically actionable with FDA-approved drugs include changes in EGFR, ALK, ROS1, MET, RET, NTRK1 / 2/3 and BRAF genes. Most of these mutations occur during a patient’s lifetime and only occur in cancer cells.
ALK fusions are seen in younger patients, nonsmokers or light smokers. When the ALK gene combines with another gene, EML4, it can trigger the development of lung cancer. ALK fusions are typically not found in patients with EGFR mutations.
ROS1 fusions are usually identified in young nonsmokers. ROS1 can combine with many other genes to create an oncogenic fusion that can lead to lung cancer.
RET Fusions:
Similar to the ALK and ROS1 fusions in lung adenocarcinoma, RET fusions can initiate and sustain the tumor.
Sources:
https://www.cancer.org/lung-cancer
https://www.ncbi.nlm.nih.gov/articles
ALK gene: MedlinePlus Genetics
ALK Mutation (Gene Rearrangement) | Lab Tests Online
Definition of solid tumor – NCI Dictionary of Cancer Terms – National Cancer Institute
What is ALK-positive lung cancer? By the ALK Positive Community — ALK POSITIVE





































Massive Bio has gone above and beyond to connect my family with the right care during Covid-19. I really appreciate their staff’s consistent and clear outreach during this process.
They have a really responsive and hard-working team. It’s a great way to explore clinical cancer trials and find alternative treatments.
Massive Bio helped me understand the importance and benefits of clinical trials, then found personalized matches using an AI database and recommended a nearby trial to enroll in. Their customer service was helpful in answering any questions about the services and process.
My father used the Virtual Tumor Board to get another treatment recommendation. The report was detailed, and the staff answered all our questions. I definitely recommend their services.
I have recommended this to colleagues for patients that could benefit from clinical trials or have exhausted their treatment options and need a second opinion. Massive Bio’s team puts great minds together to find better solutions for patients that might think they have none left.
Massive Bio’s team is amazing. They work tirelessly to help patients and providers. This company truly cares about the work they are doing and the care they are delivering.