News
Massive Bio Has Onboarded Over 120,000 Cancer Patients To Find Their Clinical Trial
Embarking on Promising Paths: Myelofibrosis Clinical Trials
Myelofibrosis often raises numerous questions, but there is newfound optimism. Our platform connects individuals to the latest clinical trials, focusing specifically on those related to myelofibrosis. We simplify the process for patients to find and participate in new medical studies, granting them access to cutting-edge treatments.
Compliant with

You can receive a guidebook with information about myelofibrosis by filling out the form.
The Latest in Myelofibrosis Treatment Options
Our matching system bridges the gap to innovative clinical trials, offering a realm of possibilities for those dealing with myelofibrosis. Within our platform, you’ll find comprehensive details about clinical trials for all phases of the disease. These trials feature new treatments designed to target the specific cause of your myelofibrosis. This gives you an opportunity to proactively combat the disease with us.
How Our System Works
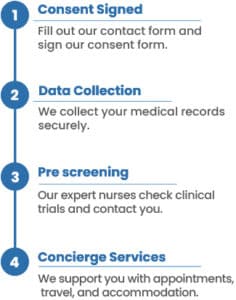
By reviewing your medical records and utilizing our AI system, we identify clinical trials you may qualify for. We also provide a concierge service to assist with your smooth transition into these trials. Our team of nurses and doctors manages all aspects of your participation from start to finish. All services are completely free for you and your oncologist.
Understanding the Disease
What is Myelofibrosis?
Myelofibrosis, a chronic myeloproliferative disorder, is a rare type of blood cancer in which normal bone marrow tissue is replaced by fibrous scar tissue (fibrosis). Since the bone marrow’s primary role is to produce red blood cells, white blood cells, and platelets, fibrosis disrupts the production of these cells, leading to various symptoms and complications.
Classification of Myelofibrosis
Myelofibrosis is classified into two main types:
- Primary Myelofibrosis: This form develops independently, without any preceding blood disorder. A genetic mutation in bone marrow cells causes too many cells to grow and inflammation, leading to fibrosis. Scar tissue forms in the bone marrow, reducing its function and leading to fewer blood cells of all types (pancytopenia).
- Secondary Myelofibrosis: This is a complication of other blood disorders. One example is essential thrombocythemia (Post ET Myelofibrosis), which causes an excess of platelets. Another example is polycythemia vera (Post Polycitemia Vera Myelofibrosis), which results in too many red blood cells. It evolves from an existing condition, where the bone marrow gradually becomes fibrotic, leading to symptoms and complications similar to those of primary myelofibrosis.
What are the Common Symptoms of Myelofibrosis?
- Severe Fatigue: Persistent tiredness because of anemia, affecting up to 50% of patients.
- Abdominal Discomfort: A heavy sensation in the left upper abdomen, often because of an enlarged spleen.
- Enlarged Spleen (Splenomegaly): The spleen becomes enlarged due to the accumulation of abnormal blood cells and fibrosis. This can lead to pain or a feeling of fullness in the abdomen, especially after eating small amounts.
- Unintentional Weight Loss: Significant weight loss without trying, commonly associated with disease progression.
- Bone Pain: Pain in the bones, especially in the legs or ribs, because of abnormal marrow activity.
- Night Sweats: Excessive sweating during the night, often soaking clothes.
- Easy Bruising: Frequent bruising because of a low platelet count.
Recognizing myelofibrosis symptoms, such as fatigue, anemia, and an enlarged spleen, is vital for early diagnosis and effective management. Early detection allows timely treatment, which helps manage symptoms, prevent complications, and improve patient outcomes.
Myelofibrosis Risk Classification
Unlike traditional cancer staging, myelofibrosis is staged using scoring systems. The most commonly used are DIPSS (Dynamic International Prognostic Scoring System), DIPSS-Plus, and MIPSS70+ v2.0, which is specifically designed for patients under 70 years old. These systems assess several risk factors and assign points, classifying the disease into four risk categories:
- Low Risk: Indicates the mildest form of myelofibrosis.
- Intermediate-1 Risk: Suggests moderate disease progression.
- Intermediate-2 Risk: Indicates more rapid disease progression, requiring active treatment.
- High Risk: Suggests an advanced form of myelofibrosis that requires intensive treatment.
Accurate staging helps tailor treatments to specific disease risks, optimizing efficacy and safety for myelofibrosis patients.
Treated vs. Untreated Myelofibrosis
Untreated Myelofibrosis: Refers to patients who have been diagnosed but have not yet received any specific treatment. Clinical trials for these patients often focus on novel therapies and combinations to improve initial treatment outcomes and long-term prognosis.
Treated Myelofibrosis: Refers to patients who have already undergone treatment but may have experienced disease progression, relapse, or insufficient response. Clinical trials for this group focus on new treatment options for relapsed, refractory, or treatment-intolerant patients.
Clinical trials are available for myelofibrosis patients. This includes both those who have been treated and those who have not. These trials offer new therapies that may improve disease management and outcomes.
Stem Cell Transplant Eligible vs. Ineligible
After diagnosing myelofibrosis and assessing risk, it’s crucial to determine eligibility for hematopoietic stem cell transplantation (HCT). HCT is a procedure where a patient’s damaged bone marrow is replaced with healthy blood-forming stem cells from a suitable donor.
- Stem Cell Transplant Eligible: Typically offered to patients under 70 years old without major comorbidities, diagnosed with high-risk myelofibrosis resistant to JAK2 inhibitors or other non-transplant treatments.
- Stem Cell Transplant Ineligible: Patients with mild symptoms related to myelofibrosis may not be eligible, as the adverse effects of the transplant could outweigh the benefits compared to existing treatments.
Clinical trials are available for myelofibrosis patients, whether they are eligible or ineligible for stem cell transplants. These trials offer new therapies and treatment strategies to improve transplant outcomes, manage symptoms, and slow disease progression, expanding care options for all patients.
What are the Current Options for Myelofibrosis?
This is a common question, and the answers are continuously evolving. Myelofibrosis has different subtypes, with Primary Myelofibrosis being one of the most common. Treatment approaches vary based on the disease stage, with more advanced stages often requiring a combination of therapies.
Early detection of myelofibrosis can be challenging, but it can lead to a quicker diagnosis and potentially improve treatment outcomes.
- JAK Inhibitors: These drugs target and inhibit the overactive JAK protein in myelofibrosis, reducing inflammation, spleen size, and abnormal blood cell production.
- Hydroxyurea: This drug reduces the overproduction of blood cells and controls symptoms like splenomegaly in patients who are ineligible for stem cell transplantation or JAK inhibitors.
- Splenectomy is surgery to remove the spleen. This procedure is done for enlarged spleen treatment when causes serious pain or when other treatments have not been effective.
- Hematopoietic Cell Transplantation: Typically reserved for younger patients or those with high-risk disease due to the procedure’s risks.
- EPO-stimulating Agents help the bone marrow produce more red blood cells. They are used to treat anemia due to Myelofibrosis.
- Blood Transfusions: For individuals with severe anemia because of myelofibrosis, regular blood transfusions may be necessary to maintain healthy hemoglobin levels.
NGS Testing and Clinical Trials
Next-Generation Sequencing (NGS) is essential for linking patients with specific genetic mutations in their cancers to clinical trials for targeted treatments. This approach, part of personalized medicine, increases the effectiveness and success rates of these trials by ensuring patients receive therapies most likely to work for their particular cancer type.
The genetic mutations, also known as biomarkers, are often related to the disease’s origin. Some common biomarkers associated with myelofibrosis include:
- JAK2 Mutations: Changes in the JAK2 gene, especially V617F, are common in myelofibrosis, seen in about half of patients. These mutations result in the excessive production of blood cells, contributing to the development of myelofibrosis.
- CALR: A mutated protein that interacts with the MPL receptor on hematopoietic stem cells, activating the JAK-STAT pathway and leading to cell proliferation and inflammation. It is found especially in patients without the JAK2 mutation.
- MPL Mutations: Mutations in the MPL (Myeloproliferative Leukemia Virus Oncogene) gene are less common but are still associated with myelofibrosis. MPL mutations affect the MPL receptor, which regulates blood cell production, contributing to the disease’s pathogenesis.
- Triple-Negative Status: In some myelofibrosis cases, none of the above-mentioned mutations (JAK2, CALR, or MPL) are present. These cases are termed “triple-negative.” Despite the absence of these mutations, other genetic alterations or factors may drive myelofibrosis development in these individuals. Research continues to uncover additional biomarkers to explain these cases.
- ASXL1: A mutated gene involved in DNA expression, associated with a poorer prognosis and higher risk of disease progression.
- EZH2: A mutation involved in gene expression regulation, linked to a more aggressive disease course and poor prognosis.
- SRSF2: Mutations that impair RNA splicing, leading to abnormal protein production and poor outcomes in high-risk disease.
The Role of Clinical Trials in Myelofibrosis
Clinical trials play a crucial role in the treatment of myelofibrosis for several reasons:
- Access to Innovative Therapies: Clinical trials offer individuals the opportunity to access novel treatments, particularly crucial for serious conditions like myelofibrosis, where conventional therapies may be less effective.
- Enhancing Outcomes: Participation in clinical trials allows patients to contribute valuable insights that may enhance survival rates and overall well-being for future myelofibrosis patients.
- Comprehensive Medical Attention: Patients enrolled in clinical trials typically receive comprehensive care, with a dedicated team of healthcare providers and researchers closely monitoring their progress.
- Hope for the Future: For many individuals dealing with myelofibrosis, involvement in a clinical trial instills hope, both for their own recovery and for the potential positive impact on future patients.
- Promoting Research Advancements: Clinical trials play a pivotal role in advancing research, aiding scientists in understanding the effectiveness of new treatments and identifying which patients benefit the most.
We combine the power of technology with our dedicated team of medical providers to find you the best treatment options available.
Access the free matching toolHow Massive Bio Helps Patients With Myelofibrosis
Massive Bio specializes in finding clinical trials of new treatments for MF and related conditions. If you’ve been diagnosed with MF or any other myeloproliferative neoplasm, we’re here to help. That’s true even if you require advanced myelofibrosis treatment or advanced blood cancer treatment for any other type of myeloproliferative disorder.
If your MF has progressed, let us help you find the best treatment options. Using our artificial intelligence-based platform, we can match you to clinical trials of advanced myelofibrosis treatment options today. If you aren’t sure if you have MF or a related condition, that’s okay. Additional testing can help determine your exact diagnosis.
Talk to us. Our nurses and patient relations coordinators are here and happy to help you. You are not alone in this fight.
Call +1 844 627 7246What is a Myelofibrosis clinical trial?
Clinical trials test the latest scientific advancements in Myelofibrosis treatment. Patients who choose to enroll in trials can receive cutting-edge treatment and high-quality care under the direction of scientists, doctors, and researchers. Myelofibrosis patients might gain access to promising drugs and innovative treatments long before they're made available to the public.
What Are Clinical Trials?
Cancer is an unfortunate reality that touches most of us at some point in our lives. If you or a loved one has cancer, you may have heard or read that clinical trials could offer access to innovative new treatments. But what exactly is a clinical trial? In this video, Massive Bio co-founder Arturo Loaiza-Bonilla, MD, explains how clinical trials work, what to expect if you enroll in one, and why a clinical trial can be an important treatment option for many cancer patients.
We dream of the day when cancer disappears from our lives. Massive Bio is working tirelessly on achieving that goal.
-
Why should I trust Massive Bio?
Why should I trust Massive Bio?
Massive Bio has provided health to more than 120,000 cancer patients in 25 countries across three continents. It collaborates with over 80 global partners. In 2022, Massive Bio became part of the Precision Cancer Consortium (PCC), alongside major companies such as AstraZeneca, Bayer, Eli Lilly & Company, GSK, Johnson & Johnson/Janssen, Novartis and Roche.
-
What is the process?
What is the process?
To find the best clinical research studies for you, we need your medical history and consent. You can provide this consent by filling out the form on this page and the following pages. Once you’ve done that, our patient relations coordinator will contact you to discuss the details and provide further information for clinical research matching report.
-
Why do I have to provide my medical records to enroll in a clinical trial?
Why do I have to provide my medical records to enroll in a clinical trial?
To enroll in clinical trial, you must meet highly specific criteria that’s established by the researchers who are conducting the investigation. That includes detailed information about type of cancer, treatment history, response to treatment, and other data that is collected in medical records.
-
What should I do if I don’t have my medical records?
What should I do if I don’t have my medical records?
If you are being treated for cancer or any other disease, your doctor should have a complete record of your medical care, including specific information about what form of the disease you have and what treatments you have received. Your patient relations coordinator will contact you and inform you about the details.
-
What are the costs associated with Massive Bio’s services?
What are the costs associated with Massive Bio’s services?
Massive Bio provides its services to the patients and their doctors at no cost—you won’t have to pay anything to receive a clinical-research matching report. There are no hidden costs involved.
-
How does Massive Bio protect my personal information?
How does Massive Bio protect my personal information?
Massive Bio strictly adheres to all HIPAA guidelines and international regulations focused on maintaining your privacy. We take extra measures to secure your personal information, ensuring it is protected beyond the mandatory requirements.
-
Where can I find clinical research studies in my area?
Where can I find clinical research studies in my area?
Your doctor may know of a clinical research study being conducted in your area that’s recruiting participants and is right for you. However, Massive Bio uses its artificial intelligence-powered platform to match patients to clinical research studies of treatments that give you the best chance of a positive outcome and are being conducted in a geographical location that makes sense for you.
-
Can I continue seeing my doctor or oncologist while also taking services of Massive Bio?
Can I continue seeing my doctor or oncologist while also taking services of Massive Bio?
Yes, Massive Bio keeps your doctor up to date on your status throughout your participation.






