News Massive Bio Has Onboarded Over 120,000 Cancer Patients To Find Their Clinical Trial
Novel Opportunities: Cervical Cancer Clinical Trials
Cervical cancer, in all of its types and stages, can be a frightening diagnosis. Deep dive into the world of clinical trials and the great options they can offer for your treatment.
Compliant with

You can receive a guidebook with information about cancer clinical trials by filling out the form.
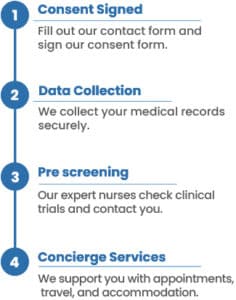
How our system works
We are here to support your search for effective cervical cancer treatments. Our state-of-the-art artificial intelligence (AI) platform can recommend personalized clinical trials, designed to suit your unique condition. We understand the importance of addressing cervical neoplasms comprehensively, and upon submitting your details, our team of oncology experts will assess your case and reach out to you.
We place particular emphasis on Stage 3 and Stage 4 (Advanced or Metastatic) cervical cancer, which are generally more difficult to treat. We simplify the process for patients to discover and participate in innovative clinical trials, providing them with opportunities to access the latest treatments.
Understanding the disease
What is cervical cancer?
It is a type of cancer that starts in the cervix of the uterus. The cells that usually line the entrance to the uterus start growing in a disorganized and uncontrolled way. These can then spread to other parts of your body if the cancer is not treated. Cervical neoplasms are classically associated to exposure to the Human Papilloma Virus (HPV); especially to its high risk subtypes.
There are two main types of cervical cancer:
1. Cervical Squamous Cell Carcinoma (SCC): This is the most common type. In the US, it accounts for 75% of all cases. It usually has better prognosis and response to the available treatment options. It is commonly associated to the HPV 16 high risk subtype.
2. Cervical adenocarcinoma: This type of cervical cancer has cells that resemble glands from the uterus. It accounts for 25% of all cases, which also includes adenosquamous tumors (which includes cell characteristics from both squamous and gland cells). This type has a higher association with the HPV 18 serotype. In general, it has a worse prognosis than cervical SCC.
Since cervical cancer’s HPV relationship is well studied, there are some of healthcare practice considerations that arise from it to screen and diagnose this cancer. Cervical cancer screening consists of a pap smear; a procedure in which cells directly taken from the cervix are looked at under a microscope for abnormalities. Likewise, HPV tests are performed to screen for high-risk HPV serotypes that can lead to cancer.
In that line, there is also an effective HPV vaccine that has been shown great results in preventing cervical cancer. In the latest years it has been under investigation to be used as a potential support in treating cervical cancer, because it activates an immune response that helps clear the virus.
Cervical cancer can travel through the body via lymph nodes and blood vessels. This progression through the body, also known as metastasis, can seed cells from the original cancer and cause new tumors in parts distant to the cervix of the uterus. In the case of this cancer type, doctors usually classify stage 3 when these new tumors are limited to the pelvic area. In contrast, when it goes to places further away in the body, like the lungs, brain or bones, it is classified as stage 4.
The symptoms of stage 4 cervical cancer may be more pronounced, including pain, fatigue, or changes in bladder and bowel habits. It’s crucial to know that stage 4 cervical cancer survival rate, and stage 4 cervical cancer life expectancy, whether with or without treatment, tend to be significantly lower than in earlier stages.
NGS testing and clinical trials
Next-generation sequencing (NGS) is an advanced technology that quickly reads the genetic code of DNA or RNA. It allows scientists to study millions of DNA pieces at the same time. It facilitates a thorough analysis of an organism’s entire genetic makeup for a more efficient understanding in a healthcare context.
Using NGS, we can match patients with specific genetic traits in their cancers to targeted clinical trials. This personalized approach boosts the success of trials by ensuring patients get treatments tailored to their cancer’s genetic profile.
The genetic mutations are also known as biomarkers. Some of them are known to be related to the origin of the disease or to changes during their progress. Common biomarkers that are related to cervical cancer are:
1. PD-L1 (Programmed Death-Ligand 1) Expression: PD-L1 is a protein that prevents the immune system from attacking normal cells in the body. However, excessive production of these in cervical cancer cells help the cells survive and keep growing out of control. Cervical neoplasms that have high levels of PD-L1 could have a better response to immunotherapy drugs that target the PD-1/PD-L1 pathway.
2. VEGF (Vascular Endothelial Growth Factor): this protein, which can be found in the surface of cancer cells, is related to the growth of new blood vessels that supply tumors. Tumor cells that express this protein could potentially have a better responses to targeted therapy drugs that work on the vascular endothelial growth factor receptor.
3. Biomarkers of HPV infection and apoptosis (E5/E6 mRNA P53): HPV infection is known to alter the normal cycle that leads to cell death. Recognizing these biomarkers can provide information regarding pathways that can be targeted by treatment.
What are cervical cancer treatment
options?
The main treatment for cervical cancer is surgery. However, this alone is only effective in early stage cervical cancer. Depending on the cancer staging, different added medications and radiation therapy schemes are added to attempt to control the disease. These include well-known drugs like carboplatin, cisplatin, fluorouracil, and, in some cases, gemcitabine.
After surgical removal, the cervical neoplasia is classified according to the risk of it having metastasis. For this, depending on how deep the cervical cancer has invaded, lymph nodes from the pelvis are also extracted and checked for cancer cells. Based on this, treatment options are decided.
Patients with advanced disease are usually treated with bevacizumab, an angiogenesis inhibitor that works by cutting off the blood supply that tumors need to grow as first line. If the tumor expresses PD-L1, pembrolizumab, a monoclonal antibody that targets the PD-L1/PD-1 pathway is also added.
For locally advanced cervical cancer, brachytherapy for cervical cancer (a type of radiation that is given directly to the affected tissues) is preferred as part of the treatment. This is usually given together with anti-cancer drugs.
The odds of survival in stage 4 cervical cancer depend heavily on the patient’s overall health, response to treatment, and the extent of the disease spread. The prognosis for stage 4 cervical cancer varies, with stage 4B cervical cancer showing the lowest survival rate due to the more widespread nature of the disease at 14.7% after 5 years.
Despite the grim statistics, there are stage 4 cervical cancer survivors, and treatment strategies aim to prolong life and alleviate symptoms. Recurrent cervical cancer is another challenging situation, where the disease reappears after a period of remission, often demanding more aggressive treatment approaches.
The Role of Clinical Trials in Cervical
Cancer
Trying New Treatments: Clinical trials give cervical cancer patients a chance to try out new treatments, especially if regular ones aren’t working well for advanced stages.
Getting Better Results: Joining a cervical cancer trial helps patients share information that could make survival rates better and improve life quality for future patients.
Good Care: People in cervical cancer trials get careful attention and close watch from a special team of healthcare experts and researchers, making sure they get thorough and supportive care.
Hope for Tomorrow: Being part of a clinical research study brings hope for those with advanced cervical cancer, not just for themselves but also for helping others in the future.
Helping Research: Cervical cancer trials are vital for research, helping scientists figure out how well new treatments work and who benefits the most. This helps create better treatment plans and personalized medicine.
We combine the power of technology with our dedicated team of medical providers to find you the best treatment options available.
How Massive Bio Helps Patients With Cervical Cancer
Massive Bio offers a quick, easy, and FREE way to find clinical trials for patients like you. With our unique clinical trial matching system and compassionate team, Massive Bio can rapidly match you to a clinical trial of a new cervical cancer.
Talk to us. Our nurses and patient relations coordinators are here and happy to help you. You are not alone in this fight.
Call +1 844 627 7246What Are Clinical Trials?
Cancer is an unfortunate reality that touches most of us at some point in our lives. If you or a loved one has cancer, you may have heard or read that clinical trials could offer access to innovative new treatments. But what exactly is a clinical trial? In this video, Massive Bio co-founder Arturo Loaiza-Bonilla, MD, explains how clinical trials work, what to expect if you enroll in one, and why a clinical trial can be an important treatment option for many cancer patients.
We dream of the day when cancer disappears from our lives. Massive Bio is working tirelessly on achieving that goal.
-
Why should I trust Massive Bio?
Why should I trust Massive Bio?
Massive Bio has provided health to more than 120,000 cancer patients in 25 countries across three continents. It collaborates with over 80 global partners. In 2022, Massive Bio became part of the Precision Cancer Consortium (PCC), alongside major companies such as AstraZeneca, Bayer, Eli Lilly & Company, GSK, Johnson & Johnson/Janssen, Novartis and Roche.
-
What is the process?
What is the process?
To find the best clinical research studies for you, we need your medical history and consent. You can provide this consent by filling out the form on this page and the following pages. Once you’ve done that, our patient relations coordinator will contact you to discuss the details and provide further information for clinical research matching report.
-
Why do I have to provide my medical records to enroll in a clinical trial?
Why do I have to provide my medical records to enroll in a clinical trial?
To enroll in clinical trial, you must meet highly specific criteria that’s established by the researchers who are conducting the investigation. That includes detailed information about type of cancer, treatment history, response to treatment, and other data that is collected in medical records.
-
What should I do if I don’t have my medical records?
What should I do if I don’t have my medical records?
If you are being treated for cancer or any other disease, your doctor should have a complete record of your medical care, including specific information about what form of the disease you have and what treatments you have received. Your patient relations coordinator will contact you and inform you about the details.
-
What are the costs associated with Massive Bio’s services?
What are the costs associated with Massive Bio’s services?
Massive Bio provides its services to the patients and their doctors at no cost—you won’t have to pay anything to receive a clinical-research matching report. There are no hidden costs involved.
-
How does Massive Bio protect my personal information?
How does Massive Bio protect my personal information?
Massive Bio strictly adheres to all HIPAA guidelines and international regulations focused on maintaining your privacy. We take extra measures to secure your personal information, ensuring it is protected beyond the mandatory requirements.
-
Where can I find clinical research studies in my area?
Where can I find clinical research studies in my area?
Your doctor may know of a clinical research study being conducted in your area that’s recruiting participants and is right for you. However, Massive Bio uses its artificial intelligence-powered platform to match patients to clinical research studies of treatments that give you the best chance of a positive outcome and are being conducted in a geographical location that makes sense for you.
-
Can I continue seeing my doctor or oncologist while also taking services of Massive Bio?
Can I continue seeing my doctor or oncologist while also taking services of Massive Bio?
Yes, Massive Bio keeps your doctor up to date on your status throughout your participation.