Synergy AI HIPAA
and Informed Consent Forms
By signing and submitting this document, I am requesting the Services from Massive Bio; I consent to Massive Bio to provide the Services to me and my healthcare provider (as required). I acknowledge that my electronic signature will result in a legally binding contract under applicable state or federal law or local laws where the Services are provided.
To Health Professionals:
I understand that filling in and signing this form permits you to give copies of all my health records, including complete General Practitioners records such as information about medicine, allergies, vaccinations, previous illnesses and test results, hospital discharge summaries, appointment letters, referral letter and any hospital records relating to my cancer diagnosis, to Massive Bio, whose details are given below.
Please give Massive Bio copies of my health records, in line with the Data Protection Act 2018, within 30 days.
HEALTH INSURANCE PORTABILITY AND ACCOUNTABILITY ACT (HIPAA) CONSENT AND RELEASE FORM
Releasing health records under the Data Protection Act 2018
Your health records
Your health records typically contain information from all consultations and contacts with health professionals and information sent to the practice about you from others, such as hospital letters.
The information they contain usually includes:
- why you saw a health professional;
- details of clinical findings and diagnoses, investigations, tests, and scans;
- any options or recommendations for care and treatment the health professional discussed with you;
- the decisions made about your care and treatment, including evidence that you agreed and details of health professionals’ actions and the outcomes.
By signing above, I am authorizing my health care provider to disclose my protected health information to Massive Bio and its subcontractor(s) to analyze for clinical trials eligibility ('Services'). I authorize the release of all medical records, treatment history, scans, and other clinical information relevant to my disease and for Massive Bio to retain my protected health information after the Services for research and maintenance of the Massive Bio research database except for records relating to mental healthcare, communicable diseases, HIV or AIDS, and treatment for alcohol or drug abuse. The undersigned individual is requesting this authorization. I understand and agree to the following:
- This authorization is voluntary, and I may refuse to sign it without affecting (1) my ability to obtain treatment from my health care provider, (2) payment for my health care, or (3) my eligibility for health care benefits.
- Any information disclosed under this authorization will be used and disclosed by Massive Bio to provide oncology clinical trials eligibility analysis services and may be no longer protected by federal or state law. Massive Bio is not a healthcare provider, and no patient-provider relationship is established through the request for this service. Treatment decisions are made at the treating physician’s discretion after an independent review of results. Massive Bio is not responsible or liable for these decisions or outcomes from prescribed treatment.
- I may revoke this authorization at any time by notifying my health care provider in writing. However, my revocation will not be effective for any action my health care provider had already taken in reliance on this authorization before it was revoked.
- Massive Bio's analysis is provided without cost to the patient or the patient's insurance.
Notes for the medical records controller
This form shows your patient's permission for you to give copies of their complete record and any hospital and other records relating to this incident to Massive Bio.
You must give Massive Bio copies of these health records unless any of the exemptions set out in Schedules 3 and 4 of the Data Protection Act 2018 apply. The main exemptions are that you must not release information that:
- is likely to cause serious physical or mental harm to the patient or another person;
- or relates to someone who would typically need to give their permission (where that person is not a health professional who has cared for the patient).
Your patient's permission for you to release information is valid only if that patient understands the consequences of their records being released and how the information will be used. The solicitor or agent named (Massive Bio) on this form must explain these issues.
Massive Bio's Representative declaration and signature
By signing this form, the client permits the release of complete health records and how the information in them may be used. This form only applies to the medical record release to Massive Bio, and separate consent will be obtained for any onward disclosures required.
To Massive Bio's Representative
Massive Bio and its representatives have told the client the implications of giving access to their health records.
Massive Bio and its representatives confirm the need for the entire health records in this case.
I (i.e., the Patient) understand that Massive Bio's DLCTMS includes these services ("Services”):
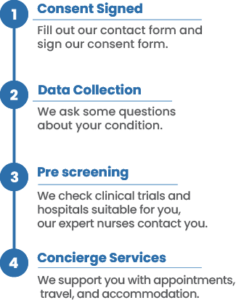
- Contact me to obtain my medical records, treatment history, scans, and clinical information relevant to my disease, followed by a review and analysis of my anonymized clinical data by Massive Bio's DLCTMS technology tool and reviewed by their team of experienced oncologists and researchers relevant to the specific disease subtype.
- Provide an analysis report to my primary oncologist that includes potential clinical trial options, diagnostic testing (e.g. next-generation sequencing) required to expand options, and current and future on-label therapeutic options required for eligibility, if applicable. Based on my primary oncologist's guidance to Massive Bio, provide the same information or the subset of the report and via real-time notifications.
- Provide my contact information (name, email, and preferred phone number) to Massive Bio partner contract research organization(s) (CRO). CRO is a company that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis.
This information would be used to facilitate enrollment in a potential clinical trial based on the DLCTMS report and analysis.
- Provide a customer support call to my primary oncologist and me to answer any relevant questions and clarifications regarding the released analysis report. My primary oncologist chooses to be present or not during the customer support call
- Finalize all customer support activities within 90 days (3 months) of the execution date of the service agreement. Massive Bio's Customer Support can be reached at [email protected] or +1-844-627-7246 if you have any questions.
I understand that Massive Bio will perform these services at no cost. I agree that I had the opportunity to review this agreement and clarify all questions before its signature.
I also understand the following disclaimers and limitations of the Services:
- Massive Bio is not practicing medicine and is not licensed, registered, certified, and otherwise qualified to practice medicine or deliver medical services in any world jurisdiction or country.
- Massive Bio is not a health care provider, and no patient-provider relationship is established by this request for services from Massive Bio.
- Massive Bio disclaims all express and implied warranties, including implied warranties of merchantability and fitness for a particular purpose. Massive Bio does not make absolute claims or representations regarding its report's accuracy, completeness, or reliability.
- Massive Bio does not provide medical advice or opinions, and our reports are advisory for educational and informational purposes only.
- Massive Bio is a healthcare data analytics firm that acts as a vendor to healthcare providers to evaluate their patient's existing clinical information and data to provide observations and information about the patient's disease subtype.
- Massive Bio does not physically observe or examine me, assess my blood, or tissue samples, or take any anatomical images for diagnostic or therapeutic purposes.
- I represent that the medical records and clinical information provided by my healthcare providers or me are accurate and complete, and Massive Bio is not responsible for, or liable to, anyone for incorrect or incomplete reporting due to inaccurate, incomplete, or unreadable clinical information received from my healthcare providers or me.
- Massive Bio will first issue its reports to my primary oncologist. Only after their review and approval will they present the report to me.
- My primary oncologist may choose to utilize or disregard the observations and information in Massive Bio's reports at their discretion.
- It is my responsibility to work with my primary oncologist to decide on clinical trials, prepare documents, and apply to clinical trials. After the report is released, if there is a specific clinical trial that I would like to enroll in, I will need to apply to the institution for the clinical trial. I will only be accepted for the clinical trial if I am eligible and my condition is suitable. Massive Bio does not guarantee acceptance or enrollment in clinical trials. Massive Bio may answer my questions regarding clinical trials and help prepare documentation as a part of customer support. However, I will communicate what I need from Massive Bio, and Massive Bio will discuss timing requirements (if applicable).
- Before engaging in a customer support call, I shall confirm my identity (name, date of birth, and zip code for identification and security purposes) and shall verbally agree to a disclaimer statement to proceed with the call.
- Massive Bio's report is the product of a proprietary knowledge base and top expert medical oncologists' input on my anonymized clinical data. Due to proprietary and confidentiality agreements, Massive Bio reserves the right not to disclose the names of the specialists who contributed to the DLCTMS development.
- After an initial review of my clinical information, if Massive Bio concludes, in its sole discretion, that the Services would not apply to my primary oncologist or me, Massive Bio may decline to process my request to analyze my anonymized clinical data through DLCTMS.
- Massive Bio will retain and use my protected health information in anonymized, aggregated form after the Services for research and maintenance of the Massive Bio research database, including the creation of Real World Data, De-Identified Data and Derivative Materials therefrom, and disclose such aggregated Real World Data, De-Identified Data, and Derivative Materials for any and all purposes (commercial, research, datasets) to its health data sharing partners.
- This Request for Services constitutes the entire agreement between Massive Bio and me concerning this subject matter and shall be construed under the laws of the United States of America and the State of Delaware. All disputes shall be resolved exclusively by binding arbitration held in New York City, United States of America.
Frequently Asked Questions
What happens if I do not sign this AUTHORIZATION form?
If you do not sign this authorization form, you will not be able to receive any of Massive Bio’s services. Signing this form is not a condition for receiving medical care, wherever you wish to pursue it.
Will I automatically be entered into a research study if I sign this form?
Without further discussion and separate consent, you cannot be entered into any research study. After the meeting, you may decide to participate in the research study; you will be asked to sign a specific research consent form.
What happens if I want to withdraw or revoke (cancel) my AUTHORIZATION?
You can change your mind and withdraw your authorization to allow your personal health information to be used in the research. If this happens, you must withdraw your cancellation in writing. Beginning on the date, you withdraw the authorization; no new personal health information will be used for research. However, researchers may continue to use the health information provided before you withdraw your authorization. To withdraw your consent, please get in touch with the person below. They will make sure your written request to withdraw your authorization is processed correctly. Clinical Research Manager RN Phone:+1-917-336-3319 Fax: +1-844-742-8837 Email: [email protected]
How long will this AUTHORIZATION last?
If you agree by signing this form that researchers can use your personal health information, this authorization has no expiration date. However, you can change your mind and withdraw your authorization, as stated above.
What are my rights regarding access to my personal health information?
You have the right to refuse to sign this authorization form. You have the right to review and copy your health information records kept by Massive Bio. You do not have the right to review and copy records, analysis, results, or any other data held by Massive Bio or other researchers associated with any research study derived from this authorization.
Signature
I agree that my personal health information may be used for any purposes described in this form.
RESEARCH SUBJECT INFORMED CONSENT FORM
Protocol Title:
SYNERGY-AI: Artificial Intelligence Based Precision Oncology Clinical Trial Matching and Registry
Sponsor:
Massive Bio, Inc (MBI)
Principal Investigator:
Selin Kurnaz, Ph.D.
Massive Bio, Inc.
90 West St. #12M,
New York, NY, 10006
+1-734-262-1020
Sub-investigators:
Ann Stevens, BSN, RN; Amanda Huttis, VP; Arturo Loaiza-Bonilla, MD MSEd; Jason Freedman MD
Why am I being asked to volunteer?
You are being asked to participate in this research study. Your participation is voluntary, so you can choose whether you want to participate. If you decide not to participate, your clinical care will not be affected.
Before agreeing to participate in this research study, it is essential that you read the following explanation of the proposed procedures and how long you will be in the study. This document describes the purpose, procedures, benefits, risks, discomforts, and study precautions. It also describes the alternative options available to you and your right to withdraw from the study.
Please take time to read the following information carefully. You may wish to discuss it with your family, friends, and doctor (i.e., your oncologist, family doctor, or primary care doctor). If you have any questions, you may ask your study doctor and the research team for more information. Take time to decide whether you wish to take part. If you choose to participate, you will be asked to sign this form. If you choose to participate, you can change your mind and withdraw from the study without giving a reason.
What is a clinical research study or clinical trial?
A clinical study (also called a trial) involves research using human volunteers (also called participants) to add to medical knowledge.
In a clinical trial, participants receive specific interventions according to the research plan or protocol created by the investigators. These interventions may be medical products, such as drugs or devices; procedures; or changes to participants' behavior, such as diet. Clinical trials may compare a new medical approach to a standard one already available, a placebo that contains no active ingredients or no intervention. Some clinical trials compare interventions that are already available to each other. When a new product or approach is being studied, it is not usually known whether it will be helpful, harmful, or no different from available alternatives (including no intervention). The investigators try to determine the safety and efficacy of the intervention by measuring certain outcomes in the participants. For example, investigators may give a drug or treatment to control high blood pressure to participating participants to see whether their blood pressure decreases.
What is the purpose of this research study? What does this study involve?
We ask your permission to include you in a research study called SYNERGY-AI: Artificial Intelligence Based Precision Oncology Clinical Trial Matching and Registry, also known as SYNERGY-AI. This project aims to review records of cancer patients and investigate whether using the data of their tumor type, past treatment history, blood laboratory workup, and the genes (DNA) of their tumors improves the chances of identifying clinical trials (investigation studies using experimental drugs). The project will investigate whether this helps the patients be eligible for a novel drug to treat their cancer, particularly after entering their information into automated matching computer software linked to a database of available clinical trials. This process will generate a list of clinical trials the patient may be eligible to enroll in based on specific characteristics ("AI clinical trial matching report").
Several experts may also review and discuss these recommendations (Virtual Tumor Board) to provide additional guidance and confirm that the patient is eligible for the selected clinical trial(s) for personalized recommendations. This knowledge may lead to better ways to prevent, detect, and treat cancer and other diseases. It is also intended to investigate the impact of matching patients to clinical trials. This project will also plan to follow your health status over time after you enroll in a study. Your cells contain your unique genes, which have the information needed to build and operate a human body. Cancer can result from changes in a person's genes that cause cells to grow uncontrollably and, sometimes, to travel to other organs. Currently, researchers and doctors know some of the genetic changes that can cause cancer, but they do not see all changes in cancer often; cancer patients are not aware they may be eligible for promising clinical trials and cancer drugs, and the overall number of patients who access clinical trials is very low.
This study is the first step in investigating if cancer patients may benefit from testing their tumor genes to look for sensitivity to certain drugs and examine if that tumor gene information can help patients enter more easily into investigational studies using those drugs targeting abnormal genes found in many cancer patients. A tumor sample was collected of your normal tissue or blood as part of your past treatment. In some cases, your oncologist (cancer doctor) may have sent the cancer tissue (and sometimes your normal tissue) for testing to find out if a change in your genes is detected. Those gene changes make cancers more susceptible to certain anti-cancer drugs. In some cases, by binding this information about genes in your cancer or normal tissue with your medical records and treatment history, it may be possible to determine if a particular change in your genes may improve your response to treatment and access to other promising therapies and studies. We are also looking to examine if using an automated software program powered by artificial intelligence (the process of automated repetitive learning and discovery through data) and a remote (virtual) evaluation of a case by a group of specialists and cancer experts may improve outcomes and the quality of cancer care. This study could lead to more knowledge about the benefit of personalized therapies, the use of computer software to match patients to studies, and the benefit of remote expert clinical decision support. With such knowledge, future treatments could become customized to a patient's unique genetic and biomarker makeup. The software could help accurately match many more patients to clinical trials in the future. It can also generalize telemedicine in cancer care and help many more patients access clinical trials and innovative therapies using a database and dedicated cancer-related software.
Who is sponsoring this study?
This study is being sponsored by Massive Bio, Inc (MBI). Your treating oncologist may participate in this study as a study investigator. MBI will collect and report study information. Tumor profiling costs (if completed) will be covered by the tumor profiling vendor selected by your doctor and your insurance (if applicable).
How do I qualify for the study?
You must be at least 18 years of age to be in the study. If you are a minor under 18 years of age, a parent or legal guardian should read and understand this consent and agree to your participation. It would help if you had a cancer diagnosis by your primary cancer doctor. If available, you must have already tried standard treatment option(s) for your type of cancer. Your organs must function well. Your doctor should agree that you can receive more treatment for your cancer. You and your primary cancer doctor should agree that looking for a clinical trial is the best next step in treating your cancer.
How many other people will be in the study?
Approximately five thousand other individuals across different centers and countries worldwide will participate in this research study.
What am I being asked to do?
We are asking your permission to obtain information from your medical records and information and results about any testing done on your cancer tissue that has been removed previously. New tissue or blood could be collected if no other sample is available to submit for cancer gene testing if that is helpful to find treatment options. Your selection (s) and medical history will help us study specification changes in genes that can better respond to some drugs and tailor your treatment into a personalized one and clinical trials you may be eligible for. We ask your permission to contact you and your primary cancer doctor several times to learn more about your health status and how you are doing with your cancer treatment. Here is how your cancer tissue testing and information will be collected and studied if you decide to participate:
- You have previously undergone surgery or biopsy to remove your cancer tissue. This tissue has been sent for gene and another marker (biomarker) testing by your cancer doctor; we ask your permission to collect those results and integrate them into a database and software.
- We ask for your permission to collect information from your medical records, including your age, ethnic background, diagnosis, geographic location, site preferences for clinical trials, insurance status, insurer’s name, disease history, and medical history and response to treatments.
What will happen to my samples and medical information?
Cancer Gene testing is usually ordered by your doctor and performed by commercially available Next Generation Sequencing Vendors (NGSV). Your blood and tissue samples contain genes and other markers which serve as "instruction books" for all the cells in your body. These NGSV laboratories analyze your genes by a method called gene sequencing. Sequencing identifies the order in which chemical compounds are arranged in your genes to see whether the order is usual or unusual. We request your permission to obtain the results of the sequencing testing done on your cancer sample and, if possible, on your normal tissue or blood sample.
The study team will collect information from your medical records, including your age, ethnic background, diagnosis, disease history, medical treatments, and response to treatments, to feed the matching algorithm software (artificial intelligence software, also known as AI) and provide recommendations for clinical trials you may be eligible for ("AI clinical trial matching report"). Several experts may also review and discuss these recommendations (Virtual Tumor Board) to provide additional advice and confirm that the patient is eligible for the selected clinical trial(s) for personalized reports. It is imperative to know that this data will be generated in a research setting and that the recommendations from the NGSV and AI are based only on a review of your medical records. All opinions stated in the generated reports are based solely on the documents received from you or your physician, without the benefit of a physical examination. Therefore, any opinions represent suggestions for testing or treatment that you or your family may choose to explore with your treating physician.
The only reports that you or your doctor will receive are related to the AI clinical trial matching and the gene sequencing data that the NGSV provides to all patients who pay for their services. Neither you nor your study doctor will be notified when research (data analysis of all patients) will be conducted. You will not get specific reports or other research specific information after receiving the AI clinical trial matching recommendations. Your anonymized data will be put together along with other participants. They could be potentially utilized for analysis by MBI and other investigators. A designated science committee at MBI will review each request. There will also be an ethics review to ensure that the request is necessary and proper. Researchers will not be given your name or any other information that could directly identify you. However, the current intent is to make research outcomes available to the broader cancer research community via publication in well-known scientific channels.
What are the possible risks or discomforts?
Risk of Using Up Stored Tumor Tissue Samples
Stored tumor tissue and blood samples may be collected for this study. These entire stored samples may be used for this research study and, therefore, may not be available for future clinical assessments as part of your routine care.
Risks of Genetic Research
This research includes genetic testing and analysis of previously performed genetic testing results. Even without your name or other identifiers, your genetic information is unique to you. The researchers believe the chance that someone will identify you is minimal, but the risk may change in the future as people come up with new ways of tracing information.
There can be a risk in knowing genetic information. During a research study, new health information about inherited traits that might affect you or your blood relatives could be found. Even though your genes are unique, you share some of the same genes with your blood relatives. Although we cannot know all the risks of participating in research on inherited traits, we believe that the chances for you and your family are very low because your samples will be coded. Research results will not be returned to you or your doctor.
Very rarely health or genetic information could be misused by employers, insurance companies, and others. For example, it could make it harder for you to get or keep a job or insurance, or life insurance companies may charge a higher rate based on this information. We believe the chance these things will happen is minimal, but we cannot make guarantees.
A federal law (Genetic Information Non-Discrimination Act, GINA) helps reduce the risk of health insurance or employment discrimination. The law does not include other types of misuse by life insurance or long-term care insurance. If you want to learn more about GINA, you can find information about it on the internet or ask the study staff.
What if new information becomes available about the study?
We may find more information that could be important to you during this study. If we discover new information about the study that could affect your decision to stay in the study, you will be notified promptly. You will be able to ask questions about this information and discuss it with your family, friends, or doctor. It is always your decision to continue your study or leave the study.
You will be told about any significant new findings or newly discovered factors during your participation in this study that may affect your health or willingness to participate. In this case, you may be asked to sign a consent form that shows that you have been informed of this new information related to this research study.
What are the possible benefits of the study?
The direct research benefit to you from participating in this study is to identify potential clinical trials you could be eligible for, matched by your tumor type, past treatment history, and cancer genetic information. There are no other direct research benefits to you from participating in this study; however, integrating cancer-specific computer software (AI) and the knowledge of several experts (Virtual Tumor Board) to provide personalized recommendations may help your treating oncologist and increase your treatment options. In addition, this study may help researchers, and health professionals better understand whether looking into the tumor's genes improves care for them, particularly after integrating the information with computer software and artificial intelligence and improving enrollment chance in clinical trials in cancer research. This may help researchers learn things that may help people in the future.
What other choices do I have if I do not participate?
Your participation in this study is entirely voluntary. Another option is not to participate in this study. Will I be paid for being in this study?
You will not be paid for taking part in this study. If any research leads to new tests, drugs, or other commercial products, there will not be any profit sharing.
Will I have to pay for anything?
If you successfully enroll in a clinical trial due to this study, any costs will be described before the informed consent for that clinical trial is considered. There are no other costs to you or your insurance associated with your participation in this study.
What happens if I am injured or hurt during the study?
We do not believe there is a possibility of any physical harm to you due to participating in this study. There are no plans for the sponsor or the Next Generation Sequencing Vendor to pay for any medical treatments as a part of this study. You will not lose any legal rights when you sign the form.
When is the Study over? Can I leave the Study before it ends?
You may stop being part of this research study for any reason. If you wish to withdraw, please get in touch with the study investigator listed on the first page of this consent form. If you withdraw, your medical records will be destroyed or returned to your hospital or doctor, and any information collected will be anonymized and de-identified. However, please understand that even if you withdraw, once your records have been coded and distributed to the participating research centers and your information transferred to the databases, it will not be possible to remove your de-identified information from this research project. If you withdraw from this project, it will in no way affect your medical care.
What if I am a non-US individual?
For former patients living outside of the UK and the EU and who once had treatment, under DPA 2018, they still have the same rights to apply for access to their health records. Such a request should be dealt with as someone making an access request from within the UK/EU set by Massive Bio terms & conditions. (https://massivebio.com/terms-and-conditions)
Who can see or use my information? How will my personal information be protected?
If you decide to participate in this study, the study investigators and staff will collect your medical and personal information to complete the survey. We will do our best to make sure that the personal information in your medical record will be kept private. However, we cannot guarantee total privacy. Your personal information may be given out if required by law. If data from this study is published or presented at scientific meetings, your name and other personal information will not be used. Regulatory agencies may review your research records. Please refer to the information below, which explains more specifically how your data is protected. If you do not want to allow these uses, you should not participate in this study. Information identifying you will be kept confidential as described below.
Why is your personal health information being used?
Your personal contact information is essential for the research team to contact you. Your personal health information is being collected as part of this research study.
What personal health information is collected and used in this study and might also be disclosed?
The following personal health information will be collected and used for this study.
- Name, address, telephone number, gender, date of birth
- The history and diagnosis of your Disease
- Specific information about the treatments you received, including previous treatment(s) you may have had
- Information about other medical conditions that may affect your treatment
- Information on side effects (adverse events) you may experience and how these were treated
- Long-term information about your general health status and your disease status. This may include information from other health care providers
- Data that may be related to tissue samples that may be collected from you
- Numbers or codes that will identify you, such as your medical record number
- After tumor profiling, o Specific somatic and germline alterations found, the allele frequency and the percentage of tumor cells within the sample
- Protein and RNA expression profile, as well as the method of assessment (if available)
While collected as part of this study by your study investigator and study team, identifying information (including your name, address, telephone number, medical record number, or any number/codes that will directly identify you) will be kept as confidential as possible. It will not be routinely disclosed outside of Massive Bio, Inc. Personal health information that could be used to identify you will not be sent to the sponsor and their designated representatives.
You will be assigned a unique subject registration number upon enrollment. This number and your initials will be used to identify you throughout this study to protect your identity. The key to this code (which links your name back to the personal health information collected during this study) will be stored in a secure area, and only the MBI and NGSV (if applicable) study team will have access to this code. However, some of the study data (e.g., date of birth) could be combined with other information to identify you. If you have questions about the specific information released, you should ask your study doctor and MBI study investigator.
What is an electronic medical record?
An Electronic Medical Record (EMR) is an electronic version of the record of your care within a health system. An EMR is simply a computerized version of a paper medical record.
Suppose you have received any services from MBI and are participating in an MBI research study. In that case, results of research-related procedures (i.e., signing consent to allow your samples to be used for research testing) may be placed in your existing EMR maintained by MBI. Once placed in your EMR, these results are accessible to appropriate MBI workforce members that are not part of the research team. Information within your EMR may also be shared with others who MBI determines the right to access your EMR (e.g., health insurance company, disability provider, etc.).
Which of our personnel may use or disclose your personal health information?
The following individuals may use or disclose your personal health information for this research study:
- The Principal Investigator and the Investigator's study team
- MBI partner contract research organization(s) (CRO). CRO is a company that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. This information would be used to facilitate enrollment in a potential clinical trial based on the SYNERGY-AI report and analysis. Authorized members of the workforce of the MBI and the Next Generation Sequencing Vendor(s), and MBI and NGSV support offices, who may need to access your information in the performance of their duties (for example: for research oversight and monitoring, to provide treatment as part of this study or as part of your routine care, to manage accounting or billing matters, etc.). This includes members of the Institutional Review Board (IRB) and an Ethics Committee at the MBI and NGSV, if applicable, responsible for reviewing and overseeing research studies to ensure safe and well managed.
Who, outside of MBI and the Next Generation Sequencing Vendor, might receive your personal health information?
As part of the study, the Principal Investigator, the study team, and others listed above may disclose your study-related records, including the research study tests and procedures results, to those listed below. This study data may be processed and transmitted using secure computer systems. In all disclosures outside of the MBI and NGSV, you will not be identified by name, medical record number, address, telephone number, or any other direct personal identifier unless disclosure of the direct identifier is required by law. In records, you will be assigned a unique code number and information disclosed outside of the MBI. NGSV, original medical records also may be reviewed by the study’s sponsor or its designated representatives, the Institutional Review Board overseeing this study (if applicable). Any of the regulatory or safety oversight organizations are outlined below. They may review these records to check data collected for the study, ensure the study is being done correctly, and analyze the results.
Individuals or organizations responsible for administering the study:
Massive Bio, Inc (MBI) (the sponsor of this study) and their designated representatives
Regulatory and safety oversight organizations
The US Food and Drug Administration (FDA) oversees the NGSV tests other regulatory agencies and their designated representatives, including international agencies, Public Health agencies, and other government agencies (including non-U.S.), as authorized or required by law. Once your personal health information is disclosed to others outside of MBI or the Next Generation Sequencing Vendor, it may no longer be covered by United States federal privacy protection regulations. The Principal Investigator or study staff will inform you if there are any additions to the list above during your active participation in the trial. Any additions will be subject to MBI and NGSV procedures developed to protect your privacy.
How long may Massive Bio and the Next Generation Sequencing Vendor be able to use or disclose your personal health information?
Your authorization to use your personal health information for this specific study does not expire. If you sign this form, we will collect your health information until the end of the research study. We may collect some information from your medical records even after you finish taking part in this study or after your death. We will keep all the information forever if we need to look at it again. We will protect this information and keep it confidential.
Your information may be held in a research database. However, MBI and the Next Generation Sequencing Vendor may not re-use or re-disclose information collected in this study for a purpose other than this study unless:
- You have given written authorization to do so
- Institutional Review Board grants permission after ensuring that appropriate privacy safeguards are in place
- As permitted by law
The data from this study may be published or used for teaching purposes. However, you will not be personally identified in any publication. Your identity will remain confidential unless disclosure is required by law.
What if you decide not to give permission to use and give out your health information?
Then you will not be able to be in this research study.
Can you change your mind?
You have the right to withdraw your permission to use your personal health information, but you must stop taking part in this study if you do so. It would help if you did so in writing to the Principal Investigator at the address on the first page. Even if you withdraw your permission, your personal health information collected before receiving your written request may still be used and disclosed as necessary for the study. If you withdraw your permission to use your personal health information, you will also be withdrawn from the research study, and no new information will be collected. However, even if you do withdraw your permission to use the data about you, we are required by the FDA and other national regulatory authorities to record anything that relates to the safety of the investigational drug under study, if applicable.
What rights do I have while applying to the data controller?
a) Learning whether personal data is processed or not,
b) If personal data has been processed, requesting information about it,
c) The purpose of processing personal data and whether the purpose of learning uses them,
d) To know the third parties to whom personal data is transferred in the country or abroad,
e) To want to correction of their data in case of incomplete or incorrect processing of personal data,
f) Deletion or destruction of personal data within the framework of the conditions stipulated in Article 7,
g) To request notification of the transactions made under subparagraphs (d) and (e) to third parties to whom personal data has been transferred,
h) Object to the emergence of a result against the person himself by analyzing the processed data exclusively through automated systems,
i) Request the compensation of the damage in case of loss due to unlawful processing of personal data
Will you be able to access your research records?
You have the right to see and get a copy of your medical records kept by MBI. However, you will not be able to review or receive some of your records related to the study until after the entire study has been completed. When the study is over, you may write to the study doctor to ask to see or copy all your medical information collected during the study. You also have the right to say how your medical information may be used and to have any incorrect data about yourself updated or corrected.
As required by US Law, a description of this clinical trial will be available at http://www.clinicaltrials.gov. This website will not include information that can identify you. At most, the website will consist of a summary of the results. You can search this website at any time.
By signing this document, you permit the MBI and the Next Generation Sequencing Vendor to use and disclose personal health information collected about you for research purposes.
Who can I call with questions, complaints, or if I'm concerned about my rights as a research subject?
If you have questions, concerns, or complaints regarding your participation in this research study or if you have any questions about your rights as a research subject, you should speak with the Principal Investigator listed on page one of this form. If a research team member cannot be reached or you want to talk to someone other than those working on the study, you may contact the Office of Regulatory Affairs at the MBI and NGSV with any questions, or concerns, or complaints by calling +1-844-627-7246.
Where can I get more information?
You may visit the National Cancer Institute Website at http://www.cancer.gov for more information about studies or general information about cancer. You may also call the MBI Cancer Information Service to get the same information at +1-844-627-7246. A description of this clinical trial will be available on http://www.clinicaltrials.gov. This Web site will not include information that can identify you. At most, the Web site will include a summary of the results. You can search this Web site at any time.
Will I be contacted again?
We may want to obtain additional samples or more information about your health or medical care in the future. We are just asking your permission to re-contact you if this should be needed. If you say yes, a person from the SYNERGY-AI Study will contact you in the future to ask whether you would be interested in participating in this additional research. You do not have to agree to further research when asked.
I agree that the investigator of this study (SYNERGY-AI), or their representative, may contact my physician or me to see if I wish to participate in other research in the future.
You agree to participate in this research study when you sign this form. This means that you have read the consent form, the study has been explained to you, your questions have been answered, you have had time to decide, and you have agreed to volunteer. You have been given the names of study staff that you can contact if you need assistance or any additional questions or concerns. You agree to follow all the instructions of your study doctor to the best of your ability and report any changes in your health during the study. Your signature also means that you are permitting the MBI and NGSV to use your personal health information collected about you for research purposes within our institution. You are also allowing the MBI and NGSV to disclose that personal health information to outside organizations or people involved with the operations of this study. You agree that your primary care physician can be informed about your participation in this clinical trial.