160k Patients


Clinical Trials
Searching for options for childhood cancer? Massive Bio can assist with our free clinical research matching service, providing access to pediatric cancer clinical trials.
Compliance and Security Certifications

Real patients. Real stories.
Hear from Massive Bio Patients:
“Someone is always there to walk through
the journey — super helpful and
supportive!”
Jose Luke Komacho, Myelofibrosis Cancer
Real patients. Real stories.
Hear from Massive Bio Patients:
“ Let people help you!”
Daniel White, Lung Cancer
Your fast track to the right trial
Our AI‑driven engine never stops until you succeed.
Complete our form to kick-start your trial search
We collect your medical records securely for advanced AI matching
Our expert oncology team check results and contact you

Get trials that truly fit your condition and location

Facing cancer in children can be overwhelming, but with the right medical support, children can fight back. When conventional treatments begin to lose their effectiveness, clinical trials for childhood cancer become a valuable alternative.
These trials offer new hope in the battle against the disease, potentially improving survival rates and reducing side effects. The ultimate goal is to secure a brighter, healthier future for every child.
If you’re seeking options for childhood cancer, we can help. Our advanced artificial intelligence (AI) platform recommends personalized trials through oncology and hematology clinical trials, tailored specifically to your child’s condition. Once you send the patient’s details, our children’s cancer and blood specialists will examine the case and contact you.
Precision Medicine: Tailored options, like the ones offered in clinical trials, are based on the genetic footprint of tumors. Identifying specific genetic mutations allows for targeted solutions, potentially reducing side effects and improving outcomes.
Reducing Side Effects: Efforts are ongoing to minimize the long-term side effects of chemotherapy and radiation. Researchers are exploring novel options with fewer adverse effects on growing bodies in order to avoid regular chemotherapy.
Collaborative Research: Collaborations among institutions, researchers, and international consortia have accelerated the development of new clinical trials.
Massive Bio provides the following services:
Clinical Trial Matching: Our service helps identify innovative pediatric cancer clinical trials that may be more effective or have fewer side effects than standard options. We specialize in matching pediatric cancer patients with suitable clinical trials.
Access to Experts: The platform connects families with oncology experts who specialize in pediatric cancers. This access ensures that families receive guidance from specialists who understand the nuances and complexities of childhood cancers.
Navigating Complexities: Managing this disease can be overwhelming. We support families by providing guidance, resources, and information to navigate the complexities of decisions and available options. If your child is suitable for a clinical trial, we can make this process seamless.
Childhood cancer, or pediatric cancer, refers to cancers that develop in children and adolescents, typically under the age of 18. These cancers differ from those found in adults, often involving unique types and behaviors that require specialized approaches.
The most common types of pediatric cancers can be broadly categorized into two groups:
Hematologic cancers: Also known as childhood blood cancers, these originate in the blood, bone marrow, or lymphatic system. They disrupt the normal production and function of blood cells, leading to conditions such as leukemia, lymphoma, and myeloma.
Solid Tumors: These are masses of cancerous cells that develop in body tissues or organs. Unlike hematologic cancers, solid tumors form lumps or growths in areas such as the brain, bones, muscles, or organs like the kidneys and liver.
Pediatric cancers are biologically distinct and often respond differently to options compared to adult cancers. Thankfully, new research and better options have greatly increased the number of children surviving cancer in recent years. Early diagnosis and specialized care are critical for the best outcomes.
Stages of Solid Tumors
Early (Stage 1): The tumor is small and confined to its original location.
Localized (Stage 2): The tumor has grown but is still limited to one area.
Locally Advanced (Stage 3): The cancer has spread to nearby tissues or lymph nodes.
Metastatic (Stage 4): The cancer has spread to distant parts of the body.
This is a general overview of staging classification, but it’s important to note that each type of cancer may have its own specific staging system. Children can join clinical trials at any stage of cancer. These trials provide personalized options that meet the specific needs of each child.
Treated Solid Tumor: This is a solid tumor that has been medically treated. The treatments can include surgery, radiation, chemotherapy, or targeted therapies. These treatments aim to shrink or remove the cancer cells. Clinical trials for children with treated tumors help test new treatments and track long-term results designed for their specific needs.
Untreated Solid Tumor: Refers to a solid tumor that has not yet received any form of medical treatment or intervention and remains in its original state since diagnosis. Clinical trials may be offered to pediatric patients with untreated tumors, providing access to innovative treatments as a first-line therapy designed specifically for children.
Resectable (Surgery-Eligible) Solid Tumor: A resectable solid tumor is one that can be removed through surgery. This means the tumor is located in a position that allows for complete or partial surgical removal without causing significant harm to surrounding vital structures or organs. Surgery is often considered a primary treatment option for resectable tumors.
Unresectable (Not Surgery-Eligible) Solid Tumor: An unresectable solid tumor is one that cannot be safely removed through surgery. This could be due to the tumor’s size, location, or its involvement with critical structures that make surgical removal too risky. In these cases, other treatment options such as chemotherapy, radiation, or targeted options are typically explored.
We offer clinical trials specifically tailored for children providing access to innovative options that may not be available through standard treatment options. Our team is dedicated to guiding through the entire matching process, helping them navigate the complexities of clinical trial enrollment. This support ensures that pediatric patients can explore cutting-edge treatments that could significantly improve their outcomes.
NGS stands for Next-Generation Sequencing, a DNA sequencing technology that quickly reads millions of DNA fragments simultaneously, providing a comprehensive view of a person’s genetic. This technology offers detailed genetic information about cancer cells, allowing for more personalized targeted clinical trials for pediatric cancer.
NGS testing helps identify genetic biomarkers—specific genetic mutations within cancer cells—that can guide treatment decisions. For instance, certain genetic mutations may indicate a better response to a particularly or open the door to targeted options.
NGS testing is considered part of the standard of care for most cancer types. Discuss this with your child’s oncologist to maximize their chances of participating on targeted option for childhood cancer that could benefit from this detailed genetic information.
Anaplastic Lymphoma Kinase (ALK) fusion-positive solid tumors can happen in specific types of cancers. The most recognized ALK positive tumors in pediatric patients include:
Rhabdomyosarcoma: A type of cancer that primarily affects children. It develops from soft tissues, particularly muscles, and can occur in various parts of the body. Symptoms depend on the tumor’s location, and treatment usually involves a combination of surgery, chemotherapy, and radiation therapy.
Inflammatory Myofibroblastic Tumor (IMT): A rare tumor that can occur in various parts of the body, most commonly in the lungs, abdomen, or pelvis. IMT is often considered benign but can behave like a low-grade cancer. Symptoms vary depending on the tumor’s location, and treatment typically involves surgical removal.
Neuroblastoma: A tumor that predominantly affects children, arising from cells of the nervous system. It primarily affects the adrenal glands but may occur in the abdomen, thoracic, cervical, and pelvic sympathetic ganglia.
Glial Tumors (Low and high grade): A diverse group of tumors that arise from cells in the nervous system and usually occur in children and young adults. These tumors are broadly categorized into low-grade and high-grade based on their aggressiveness.
Standard treatments typically include surgery, chemotherapy, radiation, immunotherapy, and sometimes stem cell transplants. These childhood cancer therapies aim to eliminate cancer while considering the child’s overall well-being. Emerging therapies, such as immunotherapy and targeted therapies, are at the forefront of pediatric cancer research.
Childhood Cancer Trials offer hope for better treatments, improved outcomes, and the advancement of medical knowledge. They provide a pathway to potentially more effective and tailored trials for children fighting cancer.
Clinical trials offer hope for better treatments, improved outcomes, and the advancement of medical knowledge. They provide a pathway to potentially more effective and tailored trials for children fighting cancer.
By signing up for our matching program, you’re taking an active role in exploring new possibilities and gaining access to an innovative option that can potentially transform your life.
Co-founder Massive Bio
& Chief of Hematology and Oncology St. Luke’s University
Dr. Arturo explains
What Are Clinical Trials?
If you’ve battled Multiple Myeloma without success through multiple different treatments, we understand your journey. Our advanced AI system will match and suggest a range of potential new myeloma trials selected specifically for you. Our case managers, experts in oncology informatics, will evaluate your submission and reach out to you.
Your privacy matters
At Massive Bio, every piece of health information you share is protected by industry‑leading safeguards—HIPAA, PIPEDA, GDPR, SOC 2 Type II and PCI‑DSS. Your data stays encrypted and under your control while we match you to the right trial.

Frequently Asked Questions
To enroll in clinical trial, you must meet highly specific criteria that’s established by the researchers who are conducting the investigation. That includes detailed information about type of cancer, treatment history, response to treatment, and other data that is collected in medical records.
If you are being treated for cancer or any other disease, your doctor should have a complete record of your medical care, including specific information about what form of the disease you have and what treatments you have received. Your patient relations coordinator will contact you and inform you about the details.
Massive Bio provides its services to the patients and their doctors at no cost—you won’t have to pay anything to receive a clinical-research matching report. There are no hidden costs involved.
Massive Bio strictly adheres to all HIPAA guidelines and international regulations focused on maintaining your privacy. We take extra measures to secure your personal information, ensuring it is protected beyond the mandatory requirements.
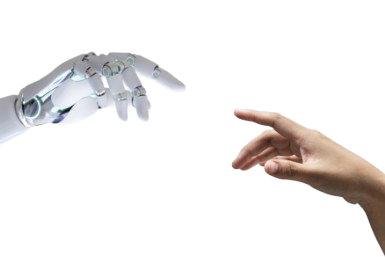
Your doctor may know of a clinical research study being conducted in your area that’s recruiting participants and is right for you. However, Massive Bio uses its artificial intelligence powered platform to match patients to clinical research studies that give you the best chance of a positive outcome and are being conducted in a geographical location that makes sense for you.
Yes, Massive Bio keeps your doctor up to date on your status throughout your participation.
Download the free MassiveBio app
Total care.
Totally different.
For iOS and Android


© 2025 Massive Bio Health, Inc. All rights reserved.
Clinical Trials
Searching for options for childhood cancer? Massive Bio can assist with our free clinical research matching service, providing access to pediatric cancer clinical trials.
Compliance and Security Certifications

Trusted worldwide by the global cancer community
Built on years of collaborative success
160k Patients
Onboarded

19k+ Trials
Matched with Patients
Trusted by 400+
Patient Advocacy Collaborations

45+
Pharmaceutical Companies

17 countries
Operating Globally

Featured in
Real patients. Real stories.
Hear from Massive Bio Patients:
“Someone is always there to walk through
the journey — super helpful and
supportive!”
Jose Luke Komacho, Myelofibrosis Cancer
Real patients. Real stories.
Hear from Massive Bio Patients:
“ Let people help you!”
Daniel White, Lung Cancer
Your fast track to the right trial
Our AI‑driven engine never stops until you succeed.
Sign & Connect
Complete our form to kick‑start
your trial search

AI‑Ready Record Sync
We collect your medical records
securely for advanced AI matching

Pre Screening
Our expert oncology team check
results and contact you

Concierge Services
We handle appointments, travel and lodging so you can focus on treatment

Facing cancer in children can be overwhelming, but with the right medical support, children can fight back. When conventional treatments begin to lose their effectiveness, clinical trials for childhood cancer become a valuable alternative.
These trials offer new hope in the battle against the disease, potentially improving survival rates and reducing side effects. The ultimate goal is to secure a brighter, healthier future for every child.
If you’re seeking options for childhood cancer, we can help. Our advanced artificial intelligence (AI) platform recommends personalized trials through oncology and hematology clinical trials, tailored specifically to your child’s condition. Once you send the patient’s details, our children’s cancer and blood specialists will examine the case and contact you.
Precision Medicine: Tailored options, like the ones offered in clinical trials, are based on the genetic footprint of tumors. Identifying specific genetic mutations allows for targeted solutions, potentially reducing side effects and improving outcomes.
Reducing Side Effects: Efforts are ongoing to minimize the long-term side effects of chemotherapy and radiation. Researchers are exploring novel options with fewer adverse effects on growing bodies in order to avoid regular chemotherapy.
Collaborative Research: Collaborations among institutions, researchers, and international consortia have accelerated the development of new clinical trials.
Massive Bio provides the following services:
Clinical Trial Matching: Our service helps identify innovative pediatric cancer clinical trials that may be more effective or have fewer side effects than standard options. We specialize in matching pediatric cancer patients with suitable clinical trials.
Access to Experts: The platform connects families with oncology experts who specialize in pediatric cancers. This access ensures that families receive guidance from specialists who understand the nuances and complexities of childhood cancers.
Navigating Complexities: Managing this disease can be overwhelming. We support families by providing guidance, resources, and information to navigate the complexities of decisions and available options. If your child is suitable for a clinical trial, we can make this process seamless.
Childhood cancer, or pediatric cancer, refers to cancers that develop in children and adolescents, typically under the age of 18. These cancers differ from those found in adults, often involving unique types and behaviors that require specialized approaches.
The most common types of pediatric cancers can be broadly categorized into two groups:
Hematologic cancers: Also known as childhood blood cancers, these originate in the blood, bone marrow, or lymphatic system. They disrupt the normal production and function of blood cells, leading to conditions such as leukemia, lymphoma, and myeloma.
Solid Tumors: These are masses of cancerous cells that develop in body tissues or organs. Unlike hematologic cancers, solid tumors form lumps or growths in areas such as the brain, bones, muscles, or organs like the kidneys and liver.
Pediatric cancers are biologically distinct and often respond differently to options compared to adult cancers. Thankfully, new research and better options have greatly increased the number of children surviving cancer in recent years. Early diagnosis and specialized care are critical for the best outcomes.
Stages of Solid Tumors
Early (Stage 1): The tumor is small and confined to its original location.
Localized (Stage 2): The tumor has grown but is still limited to one area.
Locally Advanced (Stage 3): The cancer has spread to nearby tissues or lymph nodes.
Metastatic (Stage 4): The cancer has spread to distant parts of the body.
This is a general overview of staging classification, but it’s important to note that each type of cancer may have its own specific staging system. Children can join clinical trials at any stage of cancer. These trials provide personalized options that meet the specific needs of each child.
Treated Solid Tumor: This is a solid tumor that has been medically treated. The treatments can include surgery, radiation, chemotherapy, or targeted therapies. These treatments aim to shrink or remove the cancer cells. Clinical trials for children with treated tumors help test new treatments and track long-term results designed for their specific needs.
Untreated Solid Tumor: Refers to a solid tumor that has not yet received any form of medical treatment or intervention and remains in its original state since diagnosis. Clinical trials may be offered to pediatric patients with untreated tumors, providing access to innovative treatments as a first-line therapy designed specifically for children.
Resectable (Surgery-Eligible) Solid Tumor: A resectable solid tumor is one that can be removed through surgery. This means the tumor is located in a position that allows for complete or partial surgical removal without causing significant harm to surrounding vital structures or organs. Surgery is often considered a primary treatment option for resectable tumors.
Unresectable (Not Surgery-Eligible) Solid Tumor: An unresectable solid tumor is one that cannot be safely removed through surgery. This could be due to the tumor’s size, location, or its involvement with critical structures that make surgical removal too risky. In these cases, other treatment options such as chemotherapy, radiation, or targeted options are typically explored.
We offer clinical trials specifically tailored for children providing access to innovative options that may not be available through standard treatment options. Our team is dedicated to guiding through the entire matching process, helping them navigate the complexities of clinical trial enrollment. This support ensures that pediatric patients can explore cutting-edge treatments that could significantly improve their outcomes.
NGS stands for Next-Generation Sequencing, a DNA sequencing technology that quickly reads millions of DNA fragments simultaneously, providing a comprehensive view of a person’s genetic. This technology offers detailed genetic information about cancer cells, allowing for more personalized targeted clinical trials for pediatric cancer.
NGS testing helps identify genetic biomarkers—specific genetic mutations within cancer cells—that can guide treatment decisions. For instance, certain genetic mutations may indicate a better response to a particularly or open the door to targeted options.
NGS testing is considered part of the standard of care for most cancer types. Discuss this with your child’s oncologist to maximize their chances of participating on targeted option for childhood cancer that could benefit from this detailed genetic information.
Anaplastic Lymphoma Kinase (ALK) fusion-positive solid tumors can happen in specific types of cancers. The most recognized ALK positive tumors in pediatric patients include:
Rhabdomyosarcoma: A type of cancer that primarily affects children. It develops from soft tissues, particularly muscles, and can occur in various parts of the body. Symptoms depend on the tumor’s location, and treatment usually involves a combination of surgery, chemotherapy, and radiation therapy.
Inflammatory Myofibroblastic Tumor (IMT): A rare tumor that can occur in various parts of the body, most commonly in the lungs, abdomen, or pelvis. IMT is often considered benign but can behave like a low-grade cancer. Symptoms vary depending on the tumor’s location, and treatment typically involves surgical removal.
Neuroblastoma: A tumor that predominantly affects children, arising from cells of the nervous system. It primarily affects the adrenal glands but may occur in the abdomen, thoracic, cervical, and pelvic sympathetic ganglia.
Glial Tumors (Low and high grade): A diverse group of tumors that arise from cells in the nervous system and usually occur in children and young adults. These tumors are broadly categorized into low-grade and high-grade based on their aggressiveness.
Standard treatments typically include surgery, chemotherapy, radiation, immunotherapy, and sometimes stem cell transplants. These childhood cancer therapies aim to eliminate cancer while considering the child’s overall well-being. Emerging therapies, such as immunotherapy and targeted therapies, are at the forefront of pediatric cancer research.
Childhood Cancer Trials offer hope for better treatments, improved outcomes, and the advancement of medical knowledge. They provide a pathway to potentially more effective and tailored trials for children fighting cancer.
Clinical trials offer hope for better treatments, improved outcomes, and the advancement of medical knowledge. They provide a pathway to potentially more effective and tailored trials for children fighting cancer.
By signing up for our matching program, you’re taking an active role in exploring new possibilities and gaining access to an innovative option that can potentially transform your life.

Dr. Arturo Loaiza-Bonilla
Co-founder Massive Bio
& Chief of Hematology and Oncology

Your privacy matters
At Massive Bio, every piece of health information you share is protected by industry‑leading safeguards—HIPAA, PIPEDA, GDPR, SOC 2 Type II and PCI‑DSS. Your data stays encrypted and under your control while we match you to the right trial.

Frequently Asked Questions
To enroll in clinical trial, you must meet highly specific criteria that’s established by the researchers who are conducting the investigation. That includes detailed information about type of cancer, treatment history, response to treatment, and other data that is collected in medical records.
If you are being treated for cancer or any other disease, your doctor should have a complete record of your medical care, including specific information about what form of the disease you have and what treatments you have received. Your patient relations coordinator will contact you and inform you about the details.
Massive Bio provides its services to the patients and their doctors at no cost—you won’t have to pay anything to receive a clinical-research matching report. There are no hidden costs involved.
Massive Bio strictly adheres to all HIPAA guidelines and international regulations focused on maintaining your privacy. We take extra measures to secure your personal information, ensuring it is protected beyond the mandatory requirements.
Your doctor may know of a clinical research study being conducted in your area that’s recruiting participants and is right for you. However, Massive Bio uses its artificial intelligence powered platform to match patients to clinical research studies that give you the best chance of a positive outcome and are being conducted in a geographical location that makes sense for you.
Yes, Massive Bio keeps your doctor up to date on your status throughout your participation.
Cancer Types
Clinical Trial Tools
Patients & Caregivers
Physicians

© 2025 MassiveBio Health, Inc. All rights reserved.