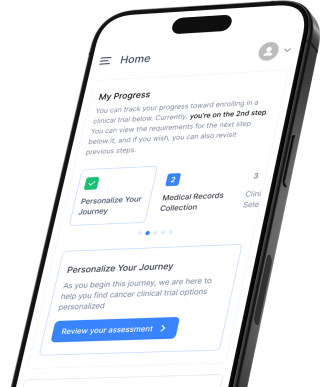
Empowers you to quickly navigate your clinical trial journey anytime, anywhere, all in one place
FOR PATIENTS AND CAREGIVERS
We connect cancer patients with clinical trials, regardless of their location or finances, using an AI-powered platform that brings together physicians, drug developers, hospitals, and genomic companies.
Just follow these 3 easy steps and talk to an expert.
Explore our AI powered technology solutions transforming cancer care.
Empowers you to quickly navigate your clinical trial journey anytime, anywhere, all in one place

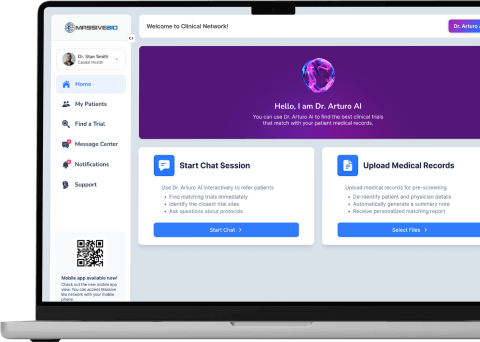
Quickly find, screen and refer your patients to the right trials with Clinical Network